Международный эндокринологический журнал Том 13, №3, 2017
Особливості біохімічних показників крові та цитокінового профілю у хворих на неалкогольну жирову хворобу печінки залежно від форми гіпотиреозу
Авторы: V.P. Prysyazhnyuk, O.I. Voloshyn, N.V. Pashkovska
Higher State Education Institution of Ukraine “Bukovinian State Medical University”, Chernivtsi, Ukraine
Рубрики: Эндокринология
Разделы: Справочник специалиста
Резюме
Мета роботи: вивчити особливості біохімічних показників крові та цитокінового профілю у хворих на неалкогольну жирову хворобу печінки (НАЖХП) залежно від форми гіпотиреозу. Матеріали та методи. Обстежено 188 хворих на НАЖХП (середній вік — 53,6 ± 12,34 року). Серед обстежених у 20 хворих на НАЖХП, окрім захворювання печінки, був діагностований субклінічний гіпотиреоз, у 24 пацієнтів — маніфестний гіпотиреоз. У групу порівняння увійшли 144 пацієнти із НАЖХП із нормальною функціональною активністю щитоподібної залози. Контрольну групу становили 45 практично здорових осіб, репрезентативних за віком та статтю щодо пацієнтів досліджуваних груп. У крові обстежених пацієнтів та практично здорових осіб досліджували біохімічні показники, визначали рівень фактора некрозу пухлин α, інтерлейкіну-10, лептину, адипонектину. Результати. Установлено, що активність загальної лактатдегідрогенази в крові хворих на НАЖХП із субклінічним та маніфестним гіпотиреозом перевищувала таку в пацієнтів із НАЖХП та нормальною функціональною активністю щитоподібної залози на 13,5 % (p = 0,02 і p = 0,01 відповідно). У хворих із поєднанням НАЖХП та маніфестного гіпотиреозу зафіксовано вірогідно вищу активність лужної фосфатази (на 12,0 %, p = 0,03) порівняно з пацієнтами групи порівняння. Концентрація лептину в крові в пацієнтів із НАЖХП як із субклінічним, так і з маніфестним гіпотиреозом перевищувала таку у хворих на НАЖХП із нормальною функціональною активністю щитоподібної залози на 35,7 % (p = 0,04) та 72,1 % (p = 0,009) відповідно. Рівень адипонектину в крові у хворих на НАЖХП та маніфестний гіпотиреоз був вірогідно меншим, ніж у пацієнтів із НАЖХП і нормальною функціональною активністю щитоподібної залози та хворих на субклінічний гіпотиреоз, у 2,1 раза (p = 0,004) та на 50,0 % (p = 0,009) відповідно. При поєднанні НАЖХП та маніфестного гіпотиреозу у хворих виявляли вірогідно більший вертикальний розмір печінки по середньоключичній лінії в середньому на 9,1 мм (p = 0,004) порівняно з пацієнтами з НАЖХП та незміненою функціональною активністю щитоподібної залози та на 8,6 мм (p = 0,04) порівняно з хворими на НАЖХП та субклінічний гіпотиреоз. Висновки. У хворих на НАЖХП із субклінічним та маніфестним гіпотиреозом спостерігаються вища активність загальної лактатдегідрогенази та вищий рівень лептину, а в останніх — ще й більша активність лужної фосфатази та нижчий рівень адипонектину в крові порівняно з відповідними показниками у хворих на НАЖХП та нормальною функціональною активністю щитоподібної залози. За наявності маніфестного гіпотиреозу у хворих на НАЖХП відмічається вірогідне збільшення вертикального розміру печінки по середньоключичній лінії порівняно з таким у пацієнтів із нормальним функціональним станом щитоподібної залози чи субклінічним гіпотиреозом.
Цель работы: изучить особенности биохимических показателей крови и цитокинового профиля у больных неалкогольной жировой болезнью печени (НАЖБП) в зависимости от формы гипотиреоза. Материалы и методы. Обследовано 188 больных НАЖБП (средний возраст — 53,6 ± 12,34 года). Среди обследованных у 20 больных НАЖБП, кроме заболевания печени, был диагностирован субклинический гипотиреоз, у 24 пациентов — манифестный гипотиреоз. В группу сравнения вошли 144 пациента с НАЖБП с нормальной функциональной активностью щитовидной железы. Контрольную группу составили 45 практически здоровых лиц, репрезентативных по возрасту и полу относительно пациентов исследуемых групп. В крови обследованных пациентов и здоровых лиц исследовали биохимические показатели, определяли уровень фактора некроза опухолей α, интерлейкина-10, лептина, адипонектина. Результаты. Установлено, что активность общей лактатдегидрогеназы в крови больных НАЖБП с субклиническим и манифестным гипотиреозом превышала таковую у пациентов с НАЖБП и нормальной функциональной активностью щитовидной железы на 13,5 % (p = 0,02 и p = 0,01 соответственно). У больных с сочетанием НАЖБП и манифестного гипотиреоза зафиксирована достоверно более высокая активность щелочной фосфатазы (на 12,0 %, p = 0,03) по сравнению с пациентами группы сравнения. Концентрация лептина в крови пациентов с НАЖБП как с субклиническим, так и с манифестным гипотиреозом превышала таковую у больных НАЖБП с нормальной функциональной активностью щитовидной железы на 35,7 %
(p = 0,04) и 72,1 % (p = 0,009) соответственно. Уровень адипонектина в крови у больных НАЖБП и манифестным гипотиреозом был достоверно меньше, чем у пациентов с НАЖБП и нормальной функциональной активностью щитовидной железы и больных субклиническим гипотиреозом, в 2,1 раза (p = 0,004) и на 50,0 % (p = 0,009) соответственно. При сочетании НАЖБП и манифестного гипотиреоза у больных обнаруживали достоверно больший вертикальный размер печени по среднеключичной линии в среднем на 9,1 мм (p = 0,004) по сравнению с пациентами с НАЖБП и неизменной функциональной активностью щитовидной железы и на 8,6 мм (p = 0,04) по сравнению с больными НАЖБП и субклиническим гипотиреозом. Выводы. У больных НАЖБП с субклиническим и манифестным гипотиреозом наблюдаются более высокая активность общей лактатдегидрогеназы и более высокий уровень лептина, а у последних — еще и большая активность щелочной фосфатазы и более низкий уровень адипонектина в крови по сравнению с соответствующими показателями у больных НАЖБП и нормальной функциональной активностью щитовидной железы. При наличии манифестного гипотиреоза у больных НАЖБП отмечается достоверное увеличение вертикального размера печени по среднеключичной линии по сравнению с таковым у пациентов с нормальным функциональным состоянием щитовидной железы или субклиническим гипотиреозом.
Background. The purpose of the study was to investigate the biochemical blood peculiarities and cytokine profile in non-alcoholic fatty liver disease (NAFLD) patients depending on the form of hypothyroidism. Material and methods. The study involved 188 NAFLD patients (average age 53.60 ± 12.34 years). Among the examined individuals 44 of them had diagnosed hypothyroidism in addition to NAFLD (20 patients had subclinical form and 24 patients had manifest hypothyroidism). A comparison group consisted of 144 NAFLD patients with thyroid normal functional activity. The control group consisted of 45 healthy individuals represented by their age and gender similar to the patients of the studied groups. Biochemical blood parameters, tumor necrosis factor α, interleukin-10, leptin, adiponectin blood levels were investigated in the observed patients and healthy individuals. Results. Total lactate dehydrogenase blood activity in NAFLD patients with subclinical and manifest hypothyroidism was found to be 13.5 % increased compared to the NAFLD patients with normal functional activity of the thyroid gland (p = 0.02 and p = 0.01, respectively). Higher alkaline phosphatase blood activity by 12.0 % (p = 0.03) was recorded in NAFLD and manifest hypothyroidism patients as compared to the patients with intact thyroid gland. Leptin blood concentration in NAFLD patients with subclinical as well as manifest hypothyroidism was 35.7 % and 72.1 % increased compared to NAFLD patients with normal thyroid functional activity (p = 0.04 and p = 0.009, respectively). Adiponectin blood level in NAFLD patients with manifest hypothyroidism was 2.1 lower (p = 0.004) in comparison
with NAFLD patients with thyroid normal functional activity and 50.0 % lower (p = 0.009) as compared to the NAFLD patients with subclinical hypothyroidism. NAFLD and manifest hypothyroidism patients showed greater vertical size of the liver measured by midclavicular line on average by 9.1 mm (p = 0.004) as compared to NAFLD patients with unchanged thyroid gland functional activity and by 8.6 mm (p = 0.04) in comparison with the NAFLD patients with subclinical hypothyroidism. Conclusions. There were found higher total lactate dehydrogenase activity and leptin blood level in NAFLD patients with subclinical and manifest hypothyroidism and higher alkaline phosphatase activity and lower adiponectin blood level in NAFLD patients with manifested hypothyroidism as compared
to NAFLD patients with normal functional activity of the thyroid gland. A significant increase in liver vertical size measured by midclavicular line was observed in NAFLD patients with manifest hypothyroidism as compared to the patients with normal thyroid function or subclinical hypothyroidism.
Ключевые слова
неалкогольна жирова хвороба печінки; гіпотиреоз; біохімічні порушення крові; лептин; адипонектин
неалкогольная жировая болезнь печени; гипотиреоз; биохимические нарушения крови; лептин; адипонектин
nonalcoholic fatty liver disease; hypothyroidism; biochemical blood disorders; leptin; adiponectin
Introduction
In recent decades, a significant increase in the pre–valence of nonalcoholic fatty liver disease (NAFLD) is observed, which occurs in about one third of adults in Western Europe and North America and in 15 % of Asian population [3]. The abovementioned is associated with a growing number of individuals with obesity, dyslipidemia, type 2 diabetes and metabolic syndrome. In addition to the foregoing factors the conditions with occurring NAFLD association are now actively discussed. Among them hypothyroidism plays an important role, which even in subclinical form is associated with an increased risk of NAFLD development [2, 5]. Some scientists indicate the level-dependent relationship between the blood concentration of thyroid stimulating hormone (TSH) and NAFLD progression [9]. At the same time, free thyroxine (T4) blood content inversely correlates with the degree of hepatic steatosis [10]. These associations are possible due toОсобливості біохімічних показників крові та цитокінового профілю
у хворих на неалкогольну жирову хворобу печінки залежно від форми гіпотиреозуactive influence of thyroid hormones on metabolism of lipids, carbohydrates, proteins and energy exchange [16].
A. Perra et al. studies demonstrates that triiodothyronine (T3) produces a strong inhibitory effect on fatty liver development and promotes regression of already formed steatosis [17]. T3 also increases the expression of several genes involved in the processes of lipogenesis in the liver: acyl-CoA synthetase 5 gene, fatty acids transport protein gene, glucose-6-phosphate dehydrogenase gene [4, 6]. The positive effect of T3 on hepatic steatosis is also realized through NADP-dependent sirtuin deacylase 1, which stimulates the oxidation of fatty acids in the liver [19]. The abovementioned indicates that in addition to oxidation stimulation, thyroid hormones inhibit the pathways contributing to the accumulation of lipids in the liver and stimulate the expenditure of lipids from their depot [8]. In case of their in sufficiency the conditions promoting accumulation of lipidsin the liver appear with subsequent addition of inflammatory processes in it.
The purpose of the study was to investigate the bioche–mical blood peculiarities and cytokine profile in NAFLD patients depending on the form of hypothyroidism.
Material and methods
The study involved 188 NAFLD patients (average age 53.6 ± 12.34 years). Among observed patients 102 (54.3 %) were males, 86 (45,7 %) — females. Among the examined individuals 44 of them in addition to NAFLD were diagnosed with hypothyroidism inclu–ding 20 patients with subclinical form and 24 patients with manifest hypothyroidism. 144 NAFLD patients with normal functional activity of the thyroid gland served as a comparison group. The control group consisted of 45 healthy individuals represented by their age and gender similar to the patientsof the studied groups.
Diagnosis of NAFLD was verified according to the Order of the Ministry of Public Health of Ukraine N 826 from 11.06.2014, adaptive clinical guidelines based on evidence “Nonalcoholic fatty liver disease” (2014) [1] and EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver dise–ase (2016) [5]. Diagnosis of hypothyroidism was veri–fied according to clinical recommendations for diagnosis of hypothyroidism of the European Thyroid Association (2013) [15], the American Association of Clinical Endocrinologists and the American Thyroid Association (2012) [7]. Compensation of hypothyroidism was determined on the basis of clinical symptoms and indicators of thyroid profile: TSH and free T4, T3 blood concentrations.
In order to exclude viral etiology ofliver disease all of the patients were tested on possible hepatitis B and C infections with the help of polymerase chain reaction method. In all of the examined patients potentially dangerous in take of alcohol (consumption more than 30 g of ethanol per day for males, and more than 20 g of ethanol daily for females) and prolonged administration of hepatotoxic drugs were excluded [1, 5]. It should be noted, that detailed analysis of anamnestic data of the observed patients indicated, that the consumption of alcohol drinks in lower than the above mentioned amounts, happened less than once a week, which eliminates the influence of ethanol as a possible etiological factor of liver damage.
All of the patients and healthy individuals underwent general comprehensive clinical, laboratory and instrumental diagnostic investigations. An informed consent was obtained from all the participants. Blood samples were obtained in the morning before taking meal from the antecubital vein. 5 % solution of disodium salt of ethylene diamine tetraacetate was used as an anticoagulant. Biochemical studies were performed on the blood biochemical analyzer “Accent-200” (“Cormay SA”, Poland). The range of indicators of biochemical blood analysis included: total bilirubin and its fractions, uric acid, total protein and albumin, urea, creatinine, plasma enzyme activity (aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (AP), gamma-glutamyl transferase (GGT)).
Examination of cytokine and adipokine profiles were performed on the immunoenzyme analyzer “Statfax 303/Plus” (“Awareness Technology Inc.”, USA). The plasma levelsoftumor necrosis factor α (TNF-α) (“Bender MedSystems GmbH”, Austria), interleukin-10 (Il-10) (“Bender MedSystems GmbH”, Austria), leptin (“Diagnostics Biochem Canada Inc.”, Canada), adiponectin (“BioVendor — Laboratorni medicina”, Czech Republic) were investigated in the examined patients and healthy individuals.
Type of data distribution was determined by compa–ring the arithmetic mean, median and mode, and using Shapiro-Wilkie test. To determine the statistical diffe–rences between two independent groups Mann-Whitney test was applied. P-values < 0.05 (p < 0.05) were consi–dered statistically significant.
Results
Patients with combined liver and thyroid pathologies admitted more pronounced complaints on pain and feeling of heaviness in the right subcostal area, frequent nausea, decreased overall health and greater general weakness, worse clinical course of the disease as compared to NAFLD patients with normal functional activity of the thyroid gland. An objective examination of these patients revealed frequent moderate pain and feeling of heaviness in the right subcostal area, hepatomegaly, presence of xanthomas, xanthelasmas and telangiectasis.
Investigations of biochemical blood tests showed significantly greater glucose, uric acid, urea, higher AST, ALT, GGT activities in the blood of NAFLD patients with normal functional activity of the thyroid gland as well as in NAFLD patients with thyroid hypofunction (table 1). Total LDG plasma activity in NAFLD patients with subclinical and manifest hypothyroidism prevailed proper indicator in NAFLD patients with normal functional activity of the thyroid gland by 13.5 % (p = 0.02 and p = 0.01 respectively). Significantly higher AP plasma activity by 12.0 % (p = 0.03) was observed in NAFLD patients and manifest hypothyroidism as compared to NAFLD patients and normal functional activity of the thyroid gland, indicating an increased severity of cholestasis [14]. The above-mentioned is probably associated with a decreased T4 blood concentration in NAFLD patients with manifest hypothyroidism, since it is known that thyroxin promotes relaxation of Oddi sphincter, however its decreased plasma concentration is accompanied with the sphincter hypertonicity causing the development of cholestasis [11]. Clinically these changes of the enzymic activity were reflected in frequent complaints of NAFLD patients with subclinical and especially manifest hypothyroidism on periodic bitter taste in the mouth, nausea, worse overall health, general weakness, headache, torpid course of the disease.
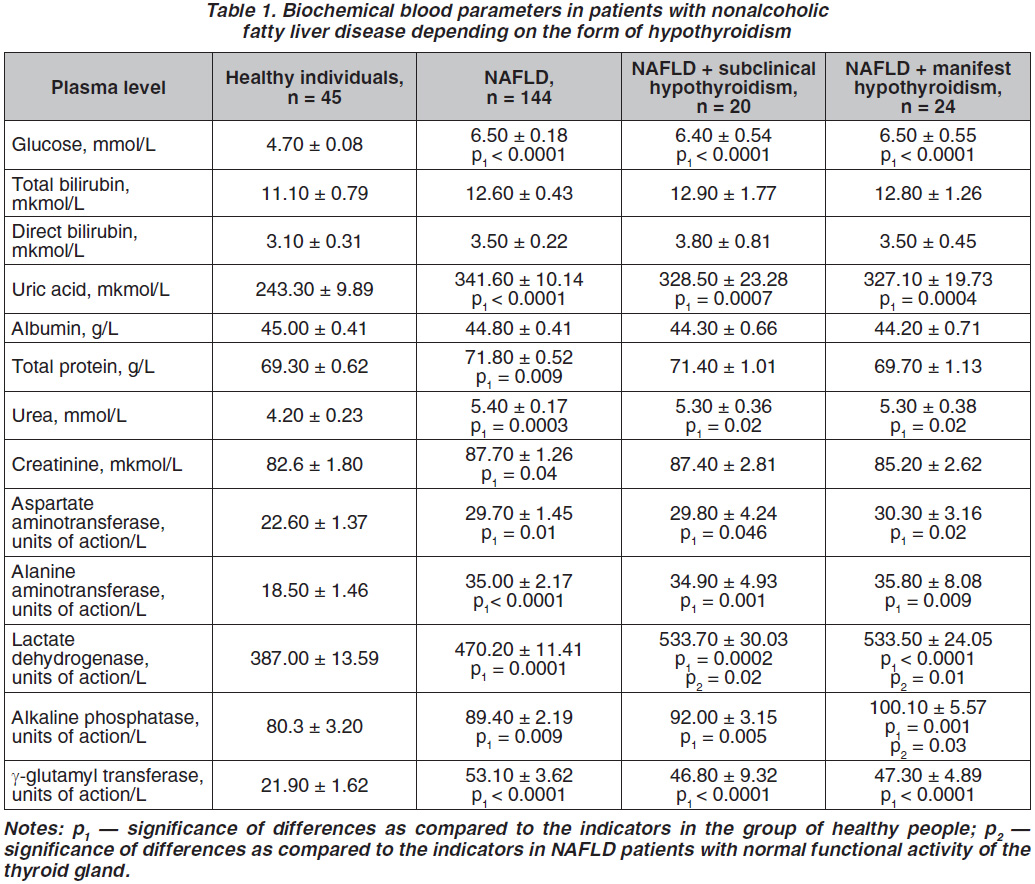
The TNF-α plasma content was more than two times higher in the examined patients of all groups as compared to healthy individuals indicating increase in the activity of inflammation processes [13]. However, the analysis of IL-10 plasma levels in the observed patients and healthy individuals did not reveal any statistically significant differences (table 2).
Leptin plasma concentration in NAFLD patients with subclinical as well as manifest hypothyroi–dism dominated over the proper parameter in NAFLD patients with normal thyroid functional activity by 35.7 % (p = 0.04) and 72.1 % (p = 0.009), respectively (table 2). The adiponectin blood level was significantly 2.1 times (p = 0.004) lower in NAFLD patients with manifest hypothyroidism as compared to patients of the comparison group. Moreover, in NAFLD patients with manifest forms of hypothyroidism adiponectin blood content was lower by 50.0 % (p = 0.009) in comparison with the proper indicator in NAFLD patients with subclinical hypothyroidism.
Discussion
Similar changes in adiponectin blood concentration in patients with various forms of NAFLD as compared to the healthy people were found by Z.M. Younossi et al. [20]. G. Li et al. demonstrated that low adiponectin blood content was associated with the progression of steatohepatitis [12], indicating an active observation and possible prophylaxis acquired in order to prevent NAFLD progression in patients with hypothyroidism. Our findings are indicative of the formation of adipokine imbalance in the observed patients, characterized by an increased leptin plasma concentration against the ground of a decreased adiponectin blood level [18].
All observed NAFLD patients both with normal functional activity of the thyroid gland and concomitant subclinical or manifest hypothyroidism determined increased right and left liver lobes sizes, which is typical for NAFLD [14]. In particular, in NAFLD patients with manifest hypothyroidism a significantly greater vertical size of the liver measured by the midclavicular line on an average 9.1 mm (p = 0.004) was observed as compared to NAFLD patients with unchanged functional activity of the thyroid gland and 8.6 mm (p = 0.04) in comparison with NAFLD patients with subclinical hypothyroidism (table 3). The mentioned enlargement of the right liver lobe was accompanied bymore pronounced sensation of heaviness in the right subcostal area, pain during liver palpation. No significant changes in the sizes of the liver left lobe in NAFLD patients of different studied groups were found.
Conclusions
1. Higher total lactate dehydrogenase plasma activity was investigated in NAFLD patients with subclinical and manifest hypothyroidism, and greater alkaline phosphatase activity in the blood — in those with manifest hypothyroidism as compared to proper indicators in NAFLD patients with normal functional activity of the thyroid gland.
2. Elevated leptin plasma level was observed in NAFLD patients with both subclinical and manifest forms of hypothyroidism, and lower adiponectin blood level — in NAFLD patients with manifest hypothyroidism in comparison with NAFLD patients with intact thyroid gland.
3. An increased vertical size of the liver right lobe was observed in NAFLD patients with manifest hypothyroidism as compared to proper parameter in NAFLD patients with normal thyroid function or subclinical hypothyroidism.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Список литературы
1. Наказ МОЗ України № 826 від 06.11.2014 «Про затвердження та впровадження медико-технологічних документів зі стандартизації медичної допомоги при хронічних неінфекційних гепатитах» / МОЗ. — К.: МОЗ, 2014. — Нормативний документ МОЗ України.
2. Паньків В.І., Юзвенко Т.Ю. Взаємозв’язок субклінічної дисфункції щитоподібної залози і метаболічного синдрому // Клінічна ендокринологія та ендокринна хірургія. — 2015. — № 3. — С. 54-59.
3. Bellentani S., Scaglioni F., Marino M. Epidemio–logy of non-alcoholic fatty liver disease // Dig. Dis. — 2010. — Vol. 28. — Р. 155-161.
4. Chou W.Y., Cheng Y.S., Ho C.L. et al. Human spot 14 protein interacts physically and functionally with the thyroid receptor // Biochem. Biophys. Res. Commun. — 2007. — Vol. 357. — Р. 133-138.
5. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease // J. Hepatol. — 2016. — Vol. 64(6). — P. 1388-1402.
6. Flores-Morales A., Gullberg H., Fernandez L. et al. Patterns of liver gene expression governed by TRbeta // Mol. Endocrinol. — 2002. — Vol. 16. — Р. 1257-1268.
7. Garber J.R., Cobin R.H., Gharib H. et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American association of clinical endocrinologists and the American thyroid association // Endocr. Pract. — 2012. — Vol. 18. — P. 988-1028.
8. Grasselli E., Voci A., Demori I. et al. 3,5-Diiodo-L-thyronine modulates the expression of genes of lipid metabolism in a rat model of fatty liver // Journal of Endocrinology. — 2012. — Vol. 2. — Р. 149-158.
9. Huang Y-Y., Gusdon A.M., Qu S. Cross-talk between the thyroid and liver: A new target for nonalcoholic fatty liver disease treatment // World J. Gastroenterol. — 2013. — Vol. 19(45). — Р. 8238-8246.
10. Ittermann T., Haring R., Wallaschofski H. et al. Inverse association between serum free thyroxine levels and hepatic steatosis: results from the Study of Health in Pomerania // Thyroid. — 2012. — Vol.6. — Р. 568-574.
11. Laukkarinen J., Sand J., Aittomäki S. et al. Mechanism of the prorelaxing effect of thyroxin on the sphincter of Oddi // Scand. J. Gastroenterol. — 2002. — Vol. 37. — P. 667-673.
12. Li G., Hu H., Shi W. et al. Elevated hematocrit in nonalcoholic fatty liver disease: a potential cause for the increased risk of cardiovascular disease? // Clinical Hemorheology and Microcirculation. — 2012. — Vol. 51. — P. 59-68.
13. Liedtke C., Trautwein C. The role of TNF and Fas dependent signaling in animal models of inflammatory liver injury and liver cancer // European Journal of Cell Biology. — 2012. –Vol. 91. — P. 582-589.
14. Mauss S., Berg T., Rockstroh J. et al. Hepatology. A clinical textbook. — 7th еd. — Hamburg, 2016. — 710 p.
15. Pearce S.H., Brabant G., Duntas L.H. et al. 2013 ETA Guideline: management of subclinical hypothyroidism // Eur. Thyroid J. — 2013. — Vol. 2(4). — P. 215-228.
16. Pergialiotis V., Konstantopoulos P., Prodromidou A. et al. Management of endocrine disease: The impact of subclinical hypothyroidism on anthropometric characteristics, lipid, glucose and hormonal profile of PCOS patients: a systematic review and meta-ana–lysis // Eur. J. Endocrinol. — 2017. — Vol. 176(3). — P. 159-166.
17. Perra A., Simbula G., Simbula M. et al. Thyroid hormone (T3) and TRbeta agonist GC-1 inhibit/reverse nonalcoholic fatty liver in rats // FASEB J. — 2008. — Vol. 22. — Р. 2981-2989.
18. Perumpail R.B., Liu A., Wong R.J. et al. Pathogenesis of hepatocarcinogenesis in non-cirrhotic nonalcoholic fatty liver disease: Potential mechanistic pathways // World Journal of Hepatology. — 2015. — Vol. 22. — P. 2384-2388.
19. Thakran S., Sharma P., Attia R.R. et al. Role of sirtuin 1 in the regulation of hepatic gene expression by thyroid hormone // J. Biol. Chem. — 2013. — Vol. 288. — Р. 807-818.
20. Younossi Z.M., Jarrar M., Nugent C.et al. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH) // Obesity Surgery. — 2008. — Vol. 18. — P. 1430-1437.