Introduction
The radiotherapy (RT) is widely used to eliminate cancer cells with fractionated external beam radiotherapy and with single-dose stereotactic body radiation therapy (SBRT). In addition to its direct effects on the cells, RT also damages the cells through the free radicals that appear at the end of the radiolysis of water, indirectly, by disrupting the atomic structures of nucleic acids, proteins, and lipids. RT also acts on cellular organelles through oxidative stress, it is known to disrupt mitochondrial functions and causes endoplasmic reticulum (ER) stress [1]. One human has 100 trillion cells. The one ml (1 cc; 1 gram) cancer tissue almost has 109 (1 000 000 000 = 1 billion) cancer cells. The solid tumor has 40–50 % hypoxic mass. In radiotherapy practice 1 cGy (1 RAD) equal to 100 erg energy transfer to 1 gram tissue. After RT the cellular death occurs by activating apoptosis, necrosis, mitotic catastrophe, aging, and autophagy (ATG) mechanisms [2]. The autophagy word takes meaning with auto (self) and phagos (to eat).
The autophagy is an important way to clean and repair the cell. Yoshinori Ohsumi was awarded the Nobel Prize in Physiology or Medicine in 2016 because of his works and discoveries on mechanisms for autophagy [3]. Autophagy is the process of removing undesired damaged cytoplasmic structures by lysosomal enzymes [4]. Autophagy is a vital process, aimed at eliminating damaged components and continuing cell survival, however, excessive stimulation causes cellular death. It is suggested that autophagy is observed in conditions such as chronic hypoxia and nutritional deficiency and plays an important role in providing metabolic hemostasis of tumor cells [5]. Low or medium-dose autophagy stimulation causes the cell to grow and regulate the cellular structure, while excessive stimulation causes cellular death due to excessive cytoplasmic loss [6]. P53 (molecular mass is in the 53 kilodalton fraction of cell proteins located on the seventeenth (17p13.1) chromosome) and PARP-1 (Poly adenosine diphosphate-ribose polymerase-1) play an important role in the initiation of ATG. Both proteins are known to inhibit mTOR (mammalian target of rapamycin) activity and cause autophagy [7].
The ATG is a catabolic mechanism that serves the damaged organelles and unnecessary long-lived proteins or toxic molecules to lysosomes for consumption, degradation, recycling, regulating homeostasis, quality control, cellular adaptation, but also cause apoptosis. The ATG is known as type-II programmed cell death. ATG starts with the membrane distention from ER or Golgi complex followed by the formation of the autophagosome. There are several ATGs (fig. 1).
If people eat a lot during life, the ATG does not work, residues and trash-dirt accumulate in the cell and these may cause an increase in cancer, Alzheimer’s, and other metabolic diseases such as Parkinson’s disease. There is cross-talk between mitochondria and proteasome system in Parkinson’s disease and the mitochondrial damage and quality control may provide with ATG and mitophagy [8]. It is better to eat less and to do detox and hunger-fasting, there is such tradition and culture in the religions.
The DNA damage observed in the acute period after RT applications activate ataxia-telangiectasia mutation (ATM) protein. The ataxia-telangiectasia mutated (ATM) protein kinase has a role in the cellular response to any hazards or radiation damage to deoxyribonucleic acid (DNA) [9]. The activation of ATM may cause the apoptosis of the damaged cell and excess ATM stimulation with Phosphatase and Tensin Homolog Induced Kinase 1 (PINK1) which destroys the cell with mitochondrial collapse [10]. The most important known mediator of DNA damage is ATM. ATM regulates autophagy by tuberous sclerosis complex 2 (TSC2) activation or Hypoxia-Inducible Factor-1alpha (HIF-1α) phosphorylation, providing mitochondrial homeostasis and quality control [11]. It is known that ATM stimulates the mitochondrial biogenesis by the way of AMP (adenosine monophosphate)-activated protein kinase (AMPK), and the energy need arising due to DNA damage is achieved, thus increasing ATP and reactive oxygen species (ROS). The reduction of mitophagy in mice with ATM deficiency is considered as an indication that ATM and mitophagy are related. The mitophagy operates with ATM, PINK1, and PARKIN.
The ATM stimulates PARKIN (Parkinson juvenile disease protein 2) via PINK 1 (PTEN-Induced Kinase 1, PTEN is Phosphatase and Tensin Homolog, and the PTEN is accepted as a tumor suppressor gene) and depolarizes the mitochondria. PARKIN is a ~52 kDa (426 amino acid) enzyme protein, encoded by Parkinson disease, parkin RBR [zinc binding domain called Really Interesting New Gene (RING)-in-between (B)-Really Interesting New Gene (RING)] E3 ubiquitin-protein ligase (PARK2) gene and located on the chromosome 6q. It has a role in the ubiquitin-proteasome system and works as a regulator of protein destroy [2]. RING-in-between-RING (RBR) E3s are a curious family of ubiquitin E3-ligases. PARK2 (also known as Parkin RBR E3 ubiquitin-protein ligase) or the PRKN (Parkin RBR E3 ubiquitin-protein ligase) gene is one of the largest genes in our genome, provides instructions for making a protein called PARKIN. As a quality control system in cells, the ubiquitin signal carries unneeded proteins into proteasomes for protein degradation. The clearance of damaged mitochondria on mitophagy became with the PINK1 accumulation on defective mitochondria [8, 12, 13].
The PINK1 and PARKIN E3 ubiquitin ligase play an important role in quality control after mitochondrial damage with mitophagy. PARKIN plays a role in the cell machinery that degrades unneeded proteins with molecules called ubiquitin. PINK1 is placed outside the damaged mitochondria membrane for mitophagy and invites PARKIN E3 ubiquitin ligase to control the defective mitochondria by isolating and destruction of the damaged site. PARKIN is normally in the cytoplasm and induces the degradation of various membrane proteins which needed to destroy by autophagy and comes to mitochondria for cleaning mitochondrial damage with mitophagy [11].
Three is “P”s of mitophagy such as PARKIN, PINK1, and post-translational modifications. Parkin mutation increases of misfolded and aggregated proteins result in mitochondria dysfunction and neurodegenerative diseases [14]. When PARKIN is mutated the Parkinson’s and many diseases such as cancer can develop, but after cancer, increased PARKIN contributes to the progression of cancer.
RT increases oxidative phosphorylation (OXPHOS) and ROS in mitochondria. ATM is the most important signal response from the nucleus to mitochondria after radiation damage. If ATM decreases, DNA damage from radiation increases, and mitochondrial response decreases. Mitochondrial damage begins with 1 Gy and after RT damage on DNA, the ATM in the nucleus, immediately increases OXPHOS and necessary energy production in mitochondria. When ROS increases, mitochondrial antioxidant glutathione is compensated for the damage, but when it eventually ends, radical and damage increase, and Parkin System tries to recover damage with mitophagy. When ATM decreases, mitophagy decreases mitochondrial damage, and stress increase. The progression of RT damage and increase of OXPHOS leads to aerobic glycolysis-Warburg effect, and lactic acid increases, which further increases mitochondria damage, initiation of cancer, genomic instability, vascular diseases, neurodegenerative diseases, aging. If any cancer, these result in cancer growth, invasion, and metastasis. ATM over Parkin provides energy, repairs, and protects the cell from apoptosis by mitophagy. If there is an ATM deficiency, radiation sensitivity increases because the mitophagy does not work and the cell dies [15].
Radiation has effects on mitochondria, plasma membrane, cytoskeleton, endoplasmic reticulum, Golgi, lysosome, nucleus, and deoxyribonucleic acid (DNA). Mitochondrial ROS cause DNA damage and genomic instability. Radiation, especially, makes more instability on mitochondrial DNA than nuclear DNA. Chronic radiation increases mitochondrial mutation. Mitochondrial dysfunction impairs cellular metabolism, causing cancer, aging, and neural diseases. With OXPHOS, superoxide anions increase in mitochondria, and subsequently, ROS increases by turning into hydrogen peroxide with manganese superoxide dismutase. Glutathione converts hydrogen peroxide (H2O2) into the water and provides protection. If there is no glutathione, excess ROS causes nucleic acid, protein, and lipid disorders.
Autophagy may save cancer, protects damaged organelles and mitochondria so that the cell does not die [16]. P53 inhibits mitophagy and leads the cell to death. Like many cancers, P53 dysfunction and hypoxia are known in lung cancers. There is radiation resistance in hypoxia. P53 is suppressed in hypoxia. P53 inhibits autophagy at the extranuclear mechanism and level [17]. P53 inhibits mitophagy. If P53 is reactivated and stimulated, mitophagy cannot occur and the radiation effect increases.
Radiation stimulates mitophagy with PINK1 and PARKIN via ATM and HIF1alfa activation by doing nuclear damage. In cases of hypoxia, radioresistance develops with mitophagy [18]. In hypoxia, P53 is inhibited and P53 function is lost, and in hypoxia, PARKIN provides cell energy, homeostasis and saves cancer. There is no hypoxia in normal tissue and there is no need for mitophagy, so P53 suppresses and balances the mitophagy
Trans Activator of Transcription HIV (Human Immunodeficiency Virus)-Transmembrane Activity of Transactivator (TAT) protein transduction domain and effect of Oxygen Dependent Degradation (ODD) domain under different oxygen tension microenvironments in vitro and in vivo. TAT-ODD (oxygen-dependent degradation)-caspase 3 under hypoxic conditions resulted in cell death by apoptosis. If the P53 effect is achieved by directing synthetic P53 peptides (HIF1 alpha with domain TAT-ODD-p53) into the hypoxic cancer cell, cancer cannot do mitophagy, and the radiation effect increases [19].
Five hours after hunger-starvation, the ATG gene opens to work and it reaches the peak level at the 10th hour. Fasting up to 72 hours cleans internally. If it takes longer, it will cause cellular damage and apoptosis. After any stress in healthy or cancer cells, this cell will decide to cellular senescence or apoptotic death or upregulation-nutrient recycling-repair-cell health revival.
Rapa-mycin takes word meaning with Rapa Nui island (Easter Island — Chile) that the investigators found a small molecule, from a soil in this island called bacterium Streptomyces Hygroscopicus which has antifungal activity. Rapamycin arrests cell or fungal activity at the G1 phase, and suppresses the immune system at G1 to S phase transition in T-lymphocytes, has immunosuppressant effect after organ transplantation, and also blocks the mTOR (mechanistic-mammalian Target Of Rapamycin). The mTOR encoded by the mTOR gene also called FRAP1 (FK506-binding protein 12-Rapamycin-Associated Protein1) member of the phosphatidylinositol 3-kinase-relate kinase family, has functioned as a serine/threonine-protein kinase, regulates, protein synthesis, cell survival, cell growth, cell proliferation, cell motility, transcription, growth factor receptors and autophagy in normal and cancer cells, such as lung cancers [20–22]. If the mTOR is active the ATG does not work, but the Rapamycin blocks the mTOR and causes ATG activation. In cancer cases, mTOR has tumor proliferation, invasion, dissemination effect and blocks DNA damage repair to cause mutation for tumorigenesis (fig. 2).
/53.jpg)
During sport, exercise, or walking on the street the ATG gene activates for optimal adaptation for muscle flexibility, condition, energy utilization and has a role to regulate cancer cachexia and sarcopenia [23–25]. The garlic, allium extracts (onion, leeks, chives, shallot, etc), and resveratrol (red grape, etc) can induce the ATG gene in the treatment of cancer and have a role to control the resistance of cancer stem cells [26–28].
Radiation decreased the mTOR phosphorylation and inactivate mTOR and activate ATG. After RT the Ataxia Telangiectasia Mutated protein (ATM) which is the sensor of DNA damage, activates the ATG gene. DNA damage repair signaling is activated by DNA lesion and direct the DDR (DNA Damage Response) pathways with phosphorylation of targets ATM, ATR (ataxia-telangiectasia and RAD3 related, RAD means Transcription factor RADIALIS) protein, p53 (gene which codes on the short (p) arm of chromosome 17 at position 13.1 (17p13.1) has 53 kilo-Dalton molecular mass), PARP1 (Poly [ADP-ribose] polymerase 1), FOXO3a (Forkhead Box O3 protein associated with Aging and Chromosome 6Q Deletion), mTOR and SIRT1 (silent mating type information regulation 2 homolog 1).
There are effects of autophagy modifications on improving radiosensitivity or radiotherapy efficacy with several ways to activate ATG after RT in the cell-related with organelles damage and molecules production such as [29]:
— PI3K-Akt-mTOR: Phosphatidylinositol-3-kinase (PI3K)-protein kinase B (Akt)-mTOR pathway;
— mitogen-activated protein kinases (MAPK): Regulate cell proliferation and survival by autophagy, which includes c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK);
— endoplasmic Reticulum (ER) Stress: PERK: Protein kinase-like endoplasmic reticulum kinase, UPR: Unfolded protein response regulate autophagy to relieve stress and re-establish cell homeostasis, ER chaperone;
— AMP-activated protein kinase (AMPK): Activated when intracellular ATP levels lower. Has roles in regulating growth and reprogramming metabolism, including autophagy and cell polarity, also ceramide and calcium, etc.
In experimental studies, the common ATG activation is through the PI3K-Akt-mTOR pathway and mTOR inhibitors have treatment effects on glioblastoma multiforme (GBM), oral, lung, breast, esophageal, and prostate cancers. The second ATG activation is through the MAPK pathway by inducing ER stress has treatment effect on pancreatic, colorectal, and prostate cancers. The third is ATG inhibition through the UPR pathway, with the addition of chloroquine for GBM and colorectal cancer [30].
The ATG activation in cancer RT is controversial. ATG; has a protection or destruction effect on cancer and we can say there is “Autophagic Switch” [31]. Because upregulation of ATG has the cancer-protective effect, inhibition of ATG has cancer destructive effect, and activation of ATG has cancer destructive effect. There are many molecules to activate (rapamycin, metformin, Bacillus Calmette Guerin-BCG etc) or inhibit (chloroquine, etc) ATG. Also, there is a relation between RT, ATG, and immunotherapy. After RT, ATG contributes to the release of cell death associated danger signals such as calreticulin exposure and HMGB1 (high mobility group box protein1) and ATP (adenosine triphosphate) release from dying cells which in turn is required for attracting immune cells including dendritic protein into the tumor that trigger antitumor host immune responses. ATG enhances the effect of immunotherapy, release antigens to dendritic cells (DC) and cytotoxic T lymphocytes, initiate an immune response (fig. 3) [32, 33].
/53_2.jpg)
Method and Results
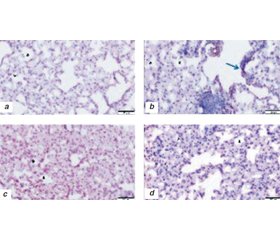
To understand the ATG effects of RT, there were some experimental studies with normal lung tissue of mice and lung cancer cell lines after RT [34, 35]. Lung tissues from 24 mice have taken with three groups × 6 = 18 mice after TBI (5 Gy) in 24 h (Group 1), 72 h (Group 2), 7 days (Group 3). The control group (6 mice) had no RT. Stained with H&E and GRP78, CHOP, MAP LC3β, and LAMP1 antibodies using immunohistochemical (IHC) technique to investigate endoplasmic reticulum (ER) stress of RT [34]. Low levels of the prosurvival protein GRP78 (ER) and increased levels of the apoptotic protein CHOP (UPR) indicated RT damage. Increased MAP LC3β (ER), LAMP1 (L) immunoreactivity indicated ATG-reticulophagy and apoptosis (fig. 4).
/54.jpg)
Also, cell proliferation capacity and clonogenicity of human NSCLC cell line A549 were determined by clonogenic assay. A549 cells and cells treated with 2, 4, 6, and 8 Gy radiation were then analyzed on days 1 and 3 after a single dose of radiotherapy. The samples of lung cancer cells were examined by H&E staining and then for ATM and PARKIN expressions were examined by IHC technique [35]. In the control groups, weak immunoreactivity of ATM and PARKIN was observed on both days 1 and 3, with the most intense ATM expression in the 6 and 8 Gy groups after day 1. The most intense PARKIN expression was seen after both day 1 and day 3 in the 2 Gy groups. PARKIN immunoreactivity decreased with increasing radiation dose. It should be considered that mitophagy (ATG) mechanisms are activated in radiotherapy (RT) applications. Understanding mitophagy in RT applications and developing targeted research may increase success in cancer treatment. It should be taken into consideration that activation of autophagic mechanisms in RT and A549 lung cancer cell line cells may provide hemostasis in cancer cells. To use with RT, molecules targeting mitophagy should be developed.
Discussion
As mentioned by Yoshinori Ohsumi the autophagy is an important way to clean and repair the cell and mitochondrial damage and quality control is provided with mitophagy. RT applications are known to cause stress in the cell due to insufficient energy supply [36]. Radiotherapy activates the vital or death mechanisms of cells as an adaptive response to cell stress. Conditions such as the metabolic environment of the cells being acidic, hypoxic, or lack of energy cause cell stress. It has been shown that acidic structures are formed in tissues where radiation is applied and this is known as a defensive mechanism [37]. It is suggested that increased lysosomal markers after radiation may be associated with autophagy [38]. Inhibition of lysosomes causes an increase in autophagosomes within the cell, creating a toxic effect [39]. Radiotherapy is widely used to eliminate cancer cells and causes oxidative stress with cell damage. However, rapid activation of ATG mechanisms can eliminate damaged cells and provide homeostasis in normal cells and also in the cancer cells [17, 18, 40, 41].
ATM is a DNA stress marker. ATM protein is the determinant of radiotherapy effect, chemotherapy effect, and lung cancer prognosis [42]. With DNA stress, ATM increases and shows that compensatory mechanisms are activated and vital functions are regulated [9]. In different dose radiation treatment groups; ATM expression in A549 cell line after 6 and 8 Gy radiation for 24 hours was not significant compared to the control group, however, the ATM immunoreactivity increased in 3 days depending on the radiation dose and was the most intense at 8 Gy [35].
The genetic inactivation of ATM is an important marker for local control success of radiotherapy in non-small cell lung cancers [43]. The increase in ATM expression indicates that DNA stress occurred with radiotherapy. There are roles of ATM in selective autophagy, including mitophagy and the tumor radiosensitivity with the functions of ATM on autophagy in cancer induced by radiation involving the MAPK14 pathway, mTOR pathway, and Beclin1/PI3KIII [44].
It has been reported that ATM immunoreactivity increases DNA stress and causes an increase in mitophagy genes such as PARKIN [10, 11]. The ATM expression was very low in lung cancer tissue belonging to the control group in which there was no DNA stress. On the other hand, in groups treated with RT, increased ATM immunoreactivity showed parallels with the application of radiation dose increase. It was thought that single-dose radiotherapy caused an increase in ATM due to DNA stress in lung cancer tissue. There is a relationship between DNA damage repair and ATM increase after lung radiotherapy. Radiation and ATM inhibition creates a radiosensitizing effect with G2 arrest and apoptosis and has therapeutic benefit in cancer tissue [45]. Still, there is no complete consensus about radiotherapy applications to eliminate the cells with the autophagy mechanism.
In addition to studies suggesting that radiotherapy is an adaptive response, especially providing cancer cells to live and grow [16, 46], studies are suggesting that excessive stimulation of autophagy causes cell death [47–50]. The ATM's expression with PARKIN parallel to 2–4 Gy is a sign that they function together in mitophagy. Low-dose RT causes an increase in mitophagy, which leads to the elimination of mitochondria-associated with expression of PARKIN. However, the decrease of PARKIN on the 1st and 3rd days with 6–8 Gy suggested that mitophagy function was completed and mitochondria collapsed.
It has been known that RT has an inflammation effect on the lung tissue [51, 52]. PARKIN expression with high-dose RT, such as stereotactic body radiotherapy (SBRT) and high dose rate endobronchial brachytherapy (EBB) [53], are considered as an indicator of mitophagy mechanisms of cells and excessive elimination of cells and/or apoptotic collapse. Radiotherapy to A549 lung cancer cell line cells causes mitochondrial damage and oxidative stress. The cell aims to continue its normal functions by eliminating damaged mitochondria with mitophagy. In this study, it was shown that “ATM-mediated mitophagy” is responsible for these effects of radiation. The cell tried to continue its normal functions with mitophagy, which aims to eliminate damaged mitochondria. It is undesirable to eliminate radiotherapy damage in cancer cells with mitophagy. Definitive radiotherapy destroys the cell with excessive ATM increase and mitochondrial collapse. Therefore, it should be taken into consideration that mitophagy mechanisms may be activated in fractional and low-dose radiotherapy applications. Understanding ATG and mitophagy in RT applications and developing targeted research may increase success in cancer treatment.
Conclusions and remarks
— In healthy people; the basal ATG is beneficial, sometimes eating less and doing detox better, and exercising (walk or sports) will lead the people healthy and long life.
— Cancer patients should not starve, should have a good and balanced diet, should not have cachexia, and their muscle mass should be protected.
— To understand ATG effects, the cancer pathologic type, tumor microenvironment (TME), hypoxia, RT fractions are important in cancer RT and for normal tissue side effects of RT. There are several experimental studies with rapamycin, chloroquine, metformin, Bacillus Calmette-Guerin (BCG), etc.
— The ATG can repair RT damage in the first 1–2 weeks of low-dose or fractionated RT. In this period if we block the ATG, the RT damages will be accumulated in cancer cells and normal tissues.
— Considering target theories, 5R/6R, alpha/beta, and RT doses after 30–50 Gy (where RT effects accumulate and peak), researches must be done and specializations on ATG effects and SWITCH time in normal and cancer cells should be developed.
— Excessive ATG and apoptosis can be expected with SBRT, SRS.
— It should be taken into consideration that ATG mechanisms may be activated in fractional and hypofractionated radiotherapy applications. Understanding ATG and mitophagy in RT applications and developing targeted molecular researchs may increase success in cancer treatment.
— Cancer can be destroyed by triggering apoptosis with the ATG over stimulation, but this may cause an increase of RT side effects.
Received 04.10.2021
Revised 19.10.2021
Accepted 27.10.2021
Список литературы
1. Chaurasia A., Bhatt A.N., Das A., Dwarakanath B.S., Sharma K. Radiation-induced autophagy: mechanisms and Consequences. Free Radical Research. 2016. 50. 273-90.
2. Golden E.B., Pellicciotta I., Demaria S., Barcellos-Hoff M.H., Formenti S.C. The convergence of radiation and immunogenic cell death signaling pathways. Front. Oncol. 2012. 2. 1-13.
3. Ohsumi Y. Scientific Background, Discoveries of Mechanisms for Autophagy. The Nobel Assembly at Karolinska Institutet. Nobelförsamlingen. 2016. 1-7.
4. Anding A.L., Baehrecke E.H. Cleaning House: Selective Autophagy of Organelles. Dev. Cell. 2017. 41. 10-22.
5. Orvedahl A., Levine B. Eating the Enemy Within: Autophagy in Infectious Diseases. Cell. Death Differ. 2009. 16. 57-69.
6. Chen N., Karantza-Wadsworth V. Role and Regulation of Autophagy in Cancer. Biochim. Biophys. Acta. 2009. 1793. 1516-23.
7. Rodriguez-Rocha H., Garcia-Garcia A., Panayiotidis M.I., Franco R. DNA Damage and Autophagy. Mutat. Res. 2011. 711(1–2). 158-66.
8. Branco D.G., Arduino D.M., Esteves A.R., Silva D.F.F., Cardoso S.M., Oliveira C.R. Cross-talk Between Mitochondria and Proteasome in Parkinson's Disease Pathogenesis. Front. Aging Neurosci. 2010. 2. 1-10.
9. Shiloh Y., Ziv Y. The ATM protein: The importance of being active. J. Cell. Biol. 2012. 198. 273-75.
10. Vit J.P., Moustacchi E., Rosselli F. ATM protein is required for radiation-induced apoptosis and acts before mitochondrial collapse. Int. J. Radiat. Biol. 2000. 76. 841-51.
11. Qi Y., Qiu Q., Gu X., Tian Y., Zhang Y. ATM Mediates Spermidine-Induced Mitophagy via PINK1 and Parkin Regulation in Human Fibroblasts. Sci. Rep. 2016. 6. 1-16.
12. Durcan T.M., Edward A., Fon E.A. The Three 'P's of Mitophagy: PARKIN, PINK1, and Post-Translational Modifications. Genes. Dev. 2015. 29. 989-99.
13. Miklya I., Göltl P., Hafenscher F. The Role of Parkin in Parkinson’s Disease. Neuropsychopharmacol. Hung. 2014. 16. 67-76.
14. Durcan T.M., Fon E.A. The Three 'P's of Mitophagy: PARKIN, PINK1, and Post-Translational Modifications. Genes. Dev. 2015. 29. 989-99.
15. Shimura T., Sasatani M., Kawai H., Kamiya K., Kobayashi J., Komatsu K., Kunugita N. ATM-mediated mitochondrial damage response triggered by nuclear DNA damage in normal human lung fibroblasts. Cell. Cycle. 2017. 16. 2345-54.
16. Chaachouay H., Ohneseit P., Toulany M., Kehlbach R., Multhoff G., Rodemann H.P. Autophagy Contributes to Resistance of Tumor Cells to Ionizing Radiation. Radiother. Oncol. 2011. 99. 287-92.
17. Vara-Perez M., Felipe-Abrio B., Agostinis P. Mitophagy in Cancer: A Tale of Adaptation. Cells. 2019. 8. 1-38.
18. Yan C., Li T.S. Dual Role of Mitophagy in Cancer Drug Resistance. Anticancer. Res. 2018. 38(2). 617-621.
19. Zheng R., Yao Q., Xie G., Du S., Ren C., Wang Y., Yuan Y. TAT-ODD-p53 Enhances the Radiosensitivity of Hypoxic Breast Cancer Cells by Inhibiting Parkin-mediated Mitophagy. Oncotarget. 2015. 6. 17417-29.
20. Laplante M., Sabatini D.M. Regulation of mTORC1 and its impact on gene expression at a glance. J. Cell. Sci. 2013. 126. 1713-19.
21. Hirsch F.R., Varella-Garcia M., Bunn P.A. Jr. et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: Correlation between gene copy number and protein expression and impact on prognosis. J. Clin. Oncol. 2003. 21. 3798-807.
22. John T., Liu G., Tsao M.S. Overview of molecular testing in non-small-cell lung cancer: Mutational analysis, gene copy number, protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors. Oncogene. 2009. 28. S14-S23.
23. Mooren F.C., Krüger K. Exercise, Autophagy, and Apoptosis. Progress in Molecular Biology and Translational Science. 2015. 135. 407-22.
24. Liang J., Zeng Z., Zhang Y., Chen N. Regulatory role of exercise-induced autophagy for sarcopenia. Exp. Gerontol. 2020. 130. 110789.
25. Gunadi J.W., Welliangan A.S., Soetadji R.S., Jasaputra D.K., Lesmana R. The Role of Autophagy Modulated by Exercise in Cancer Cachexia. Life. 2021. 11. 1-17.
26. Chu Y.L., Raghu R., Lu K.H. et al. Autophagy therapeutic potential of garlic in human cancer therapy. J. Tradit. Complement. Med. 2013. 3. 159-62.
27. Forma A., Chilimoniuk Z., Januszewski J., Sitarz R. The Potential Application of Allium Extracts in the Treatment of Gastrointestinal Cancers. Gastroenterol. Insights. 2021. 12. 136-46.
28. Tian Y., Song W., Li D., Cai L., Zhao Y. Resveratrol As A Natural Regulator of Autophagy For Prevention And Treatment Of Cancer. Onco Targets Ther. 2019. 12. 8601-9.
29. Hu L., Wang H., Huang L., Zhao Y., Wang J. Crosstalk between autophagy and intracellular radiation response. 2016. 49. 2217-26.
30. Tam S.Y., Wu V.W.C., Law H.K.W. Influence of autophagy on the efficacy of radiotherapy. Radiat. Oncol. 2017. 12. 1-10.
31. Li L., Liu W.L., Su L., Lu Z.C., He X.S. The Role of Autophagy in Cancer Radiotherapy. Curr. Mol. Pharmacol. 2020. 13. 31-40.
32. Kurtman C., Sokur I., Ozbilgin M.K. The radiotherapy might be a vaccine for immune response. Middle East Journal of Science. 2019. 1. 94-105.
33. Kurtman C., Sokur I., Zaplatina S., Martsenius O., Nesterenko T., Demchenko V., Ozbilgin M.K. Radiotherapy and Immun Response is the Radiotherapy Vaccine? Good News? Manisa Celal Bayar University J. of Inst. Health Science. 2019. 6. 199-204.
34. Uluer E.T., Kahraman G., Kılıçarslan P., Gümüştepe E., Kurtman C. Radyoterapi Uygulaması Akciğer Dokusunda Otofajiyi Artırıyor mu? Ulusal Akciğer Sağlığı Kongresi Kitabı. 2019. SS-048. P59.
35. Kurtman C., Oztatlıcı M., Ucoz M., Celik O.K., Sokur I., Ozbilgin M.K. Mitophagy in the A549 lung cancer cell line, the radiation induced damage, and the effect of ATM and PARKIN on the mitochondria. Accepted in International Journal of Radiation Research — IJRR. 2021. 316303.
36. Boyce M., Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell. Death Differ. 2006. 13. 363-73.
37. Paglin S., Hollister T., Delohery T., Hackett N., McMahill M., Sphicas E. et al., A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Canc. Res. 2001. 61. 439-44.
38. Karagounis I.V., Kalamida D., Mitrakas A., Pouliliou S., Liousia M.V., Giatromanolaki A. et al. Repression of the autophagic response sensitises lung cancer cells to radiation and chemotherapy. Br. J. Canc. 2016. 115. 312-21.
39. Solitro A.R., MacKeigan J.P. Leaving the lysosome behind: novel developments in autophagy inhibition. Future Med. Chem. 2016. 8. 73-86.
40. Mathew R., Karantza-Wadsworth V., White E. Role of autophagy in cancer. Nat. Rev. Cancer. 2007. 7. 961-7.
41. Kon M., Kiffin R., Koga H., Chapochnick J., Macian F., Varticovski L., Cuervo A.M. Chaperone-Mediated Autophagy Is Required for Tumor Growth. Sci Transl. Med. 2011. 3. 1-30.
42. Xu Y., Gao P., Lv X., Zhang L., Zhang J. The role of the ataxia telangiectasia mutated gene in lung cancer: recent advances in research. Ther. Adv. Respir. Dis. 2017. 11. 375-80.
43. Pitter K.L., Casey D.L., Setton J., Lu C., Rimner A., Reis-Filho J., Powell S.N., Lee N., Chan T.A., Riaz N. Pathogenic Mutations in ATM As Determinants of Local Control in Non-Small Cell Lung Cancers Treated with Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018. 102. S226. 1142.
44. Liang N., He Q., Liu X., Sun H. Multifaceted roles of ATM in autophagy: From nonselective autophagy to selective autophagy. Cell. Biochem. Funct. 2019. 37. 177-84.
45. Ďurišová K., Čecháková L., Jošt P., Šinkorová Z., Kmochová A., Pejchal J., Ondrej M., Vávrová J., Tichý A. DNA repair inhibitors as radiosensitizers in human lung cells. J. Appl. Biomed. 2018. 16. 66-74.
46. Kim S.H., Park E.J., Lee C.R., Chun J.N., Cho N.H., Kim I.G., Lee S., Kim T.W., Park H.H., So I., Jeon J.H. Geraniol Induces Cooperative Interaction of Apoptosis and Autophagy to Elicit Cell Death in PC-3 Prostate Cancer Cells. Int. J. Oncol. 2012. 40. 1683-90.
47. Fujiwara K., Iwado E., Mills G.B., Sawaya R., Kondo S., Kondo Y. Akt inhibitor shows anticancer and radiosensitizing effects in malignant glioma cells by inducing autophagy. Int. J. Oncol. 2007. 31. 753-60.
48. Gewirtz D.A. Autophagy as a Mechanism of Radiation Sensitization in Breast Tumor Cells. Autophagy. 2007. 3. 249-50.
49. David A., Gewirtz D.A. Autophagy, Senescence and Tumor Dormancy in Cancer Therapy. Autophagy. 2009. 5. 1232-4.
50. Kuwahara Y., Oikawa T., Ochiai Y., Roudkenar M.H., Fukumoto M., Shimura T., Ohtake Y., Ohkubo Y., Mori S., Uchiyama Y., Fukumoto M. Enhancement of Autophagy Is a Potential Modality for Tumors Refractory to Radiotherapy. Cell. Death Dis. 2011. 2. 3-11.
51. Ozbilgin M.K., Karaman G.Z., Gencur S., Gumustepe E., Kurtman C. Effects of adrenomeduline and ramp2 on the lung of mice exposed to total body radiation. Int. J. Radiat. Res. 2020. 18. 571-78.
52. Sarper B., Ozbilgin M.K., Gumustepe E., Gencur S., Karaman G.Z., Kilicaslan P., Kurtman C. PTX3 levels in murine pulmonary parenchymal tissues are correlated with radiation-induced injuries. Int. J. Radiat. Res. 2020. 18. 109-15.
53. Celebioglu B., Gurkan O.U., Erdogan S., Savas I., Köse K., Kurtman C., Gonullu U. High Dose Rate Endobronchial Brachytherapy Effectively Palliates Symptoms Due to Inoperable Lung Cancer. Jpn. J. Clin. Oncol. 2002. 32. 443-8.
/51.jpg)

/53.jpg)
/53_2.jpg)
/54.jpg)