Международный эндокринологический журнал Том 18, №3, 2022
Вернуться к номеру
Дослідження варіабельності серцевого ритму у хворих на цукровий діабет
Авторы: Srinivasa Jayachandra, Satyanath Reddy Kodidala
Department of Physiology, Zydus Medical College and Hospital, Dahod, Gujarat, India
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
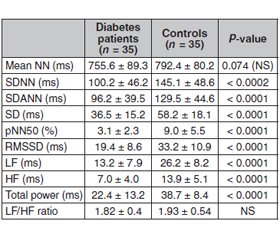
Актуальність. Варіабельність серцевого ритму (ВСР) порушена у пацієнтів з цукровим діабетом (ЦД), що свідчить про дисфункцію вегетативної регуляції серця та підвищений ризик серцевих подій. Кардіальна автономна нейропатія (КAН), яка виникає внаслідок пошкодження вегетативних нервових волокон, що іннервують серце та кровоносні судини, є серйозним ускладненням ЦД. Під час прогресування КАН парасимпатичні нервові волокна, що іннервують серце, уражаються раніше, ніж симпатичні нервові волокна, що призводить до зниження варіабельності серцевого ритму. Метою цього дослідження було обстеження пацієнтів з цукровим діабетом 2-го типу і варіабельністю серцевого ритму для діагностики вегетативної дисфункції та вивчення зв’язку отриманих даних з іншими ускладненнями цукрового діабету. Матеріали та методи. Під спостереженням перебував 41 пацієнт з ЦД 2-го типу та 45 осіб групи контролю відповідного віку і статі. Вимірювали середнє значення інтервалу R–R (NN), стандартне відхилення індексу інтервалу R–R (SDNN), стандартне відхилення 5-хвилинного інтервалу R–R (SDANN), різницю інтервалів R–R (RMSSD) і відсоток ударів з послідовною різницею інтервалів R–R > 50 мс (pNN50). У частотній ділянці вимірювали високочастотну потужність (HF), низькочастотну потужність (LF) і їх співвідношення. Результати. Не встановлено статистично значущої різниці між пацієнтами з ЦД та контрольної групи щодо розподілу за віком та статтю. Усі параметри часової та частотної ділянок, за винятком середнього інтервалу R–R та співвідношення LF/HF, були значно нижчими у пацієнтів з ЦД, ніж у групі контролю. При аналізі хронічних ускладнень ЦД, як правило, спостерігалося поєднання діабетичної ретинопатії та нефропатії. Наприклад, із шести пацієнтів з нефропатією п’ять також мали ретинопатію. Під спостереженням перебувало 13 хворих на ЦД з ускладненнями (діабетична нефропатія та/або ретинопатія) та 9 пацієнтів без ускладнень ЦД. Хоча вік хворих на цукровий діабет з ускладненнями та без них був подібним (53 ± 9 та 49 ± 12 років відповідно; P > 0,05), тривалість ЦД у пацієнтів з ускладненнями була вірогідно більшою, ніж у пацієнтів без ускладнень (14 ± 9 проти 5 ± 7 років; P = 0,002). Пацієнти з цукровим діабетом мали нижчі значення ВСР для параметрів у часовій та частотній ділянках, ніж у контрольній групі. Висновки. Більшість параметрів варіабельності серцевого ритму були нижчими у хворих на цукровий діабет із хронічними ускладненнями, ніж у пацієнтів без ускладнень.
Background. Heart rate variability (HRV) is reduced in diabetes mellitus (DM) patients, suggesting dysfunction of cardiac autonomic regulation and an increased risk for cardiac events. Cardiac autonomic neuropathy (CAN), which results from damage to autonomic nerve fibers that innervate the heart and blood vessels, is a serious complication of DM. During progression of CAN, the parasympathetic nerve fibers innervating the heart are affected before the sympathetic nerve fibers leading to a reduced heart rate variability. The purpose of this study was to examine type 2 diabetes patients with heart rate variability in order to diagnose autonomic dysfunction and to relate the findings to other complications of diabetes mellitus. Materials and methods. 41 type 2 M patients and 45 age- and sex-matched controls were included. In the time domain we measured the mean R–R interval (NN), the standard deviation of the R–R interval index (SDNN), the standard deviation of the 5-min R–R interval mean (SDANN), the root mean square of successive R–R interval differences (RMSSD) and the percentage of beats with a consecutive R–R interval difference > 50 ms (pNN50). In the frequency domain we measured high-frequency power (HF), low-frequency power (LF) and the LF/HF ratio. Results. There was no statistically significant difference between DM patients and controls for age and sex distribution. All time- and frequency-domain parameters except mean R–R interval and the LF/HF ratio were significantly lower in diabetes patients than in controls. When chronic complications of DM were examined, diabetic retinopathy and nephropathy were usually present together. For example, among six patients with nephropathy five also had retinopathy. There were 13 diabetes patients with complications (diabetic nephropathy and/or retinopathy) and nine patients with no diabetic complications. Although the chronological ages of the diabetes patients with and without complications were similar (53 ± 9 and 49 ± 12 years, respectively; P > 0.05), the duration of DM in patients with complications was significantly greater than that of those without complications (14 ± 9 versus 5 ± 7 years; P = 0.002). Diabetes patients had lower HRV values for time-domain and frequency-domain parameters than controls. Conclusions. Majority of heart rate variability parameters were lower in diabetes patients with chronic complications than in those without complications.
варіабельність серцевого ритму; цукровий діабет; діабетична ретинопатія; діабетична нефропатія
heart rate variability; diabetes mellitus; diabetic retinopathy; diabetic nephropathy
Introduction
Materials and methods
Study groups
Heart rate variability
Statistical analysis
Results
/21.jpg)
Discussion
Conclusions
- Kamenov Z.A., Traykov L.D. Diabetic autonomic neuropathy. Adv. Exp. Med. Biol. 2012. 771. 176-93. doi: 10.1007/978-1-4614-5441-0_15. PMID: 23393679.
- Freeman R. Diabetic autonomic neuropathy. Handb. Clin. Neurol. 2014. 126. 63-79. doi: 10.1016/B978-0-444-53480-4.00006-0.
- Shah A.S., El Ghormli L., Vajravelu M.E., Bacha F., Farrell R.M., Gidding S.S., Levitt Katz L.E., et al. Heart Rate Variability and Cardiac Autonomic Dysfunction: Prevalence, Risk Factors, and Relationship to Arterial Stiffness in the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study. Diabetes Care. 2019. 42(11). 2143-2150. doi: 10.2337/dc19-0993.
- Serhiyenko V.A., Serhiyenko A.A. Cardiac autonomic neuropathy: Risk factors, diagnosis and treatment. World J. Diabetes. 2018. 9(1). 1-24. doi: 10.4239/wjd.v9.i1.1.
- Bakkar N.Z., Dwaib H.S., Fares S., Eid A.H., Al-Dhaheri Y., El-Yazbi A.F. Cardiac Autonomic Neuropathy: A Progressive Consequence of Chronic Low-Grade Inflammation in Type 2 Diabetes and Related Metabolic Disorders. Int. J. Mol. Sci. 2020. 21(23). 9005. doi: 10.3390/ijms21239005.
- Spallone V. Update on the Impact, Diagnosis and Management of Cardiovascular Autonomic Neuropathy in Diabetes: What Is Defined, What Is New, and What Is Unmet. Diabetes Metab. J. 2019. 43(1). 3-30. doi: 10.4093/dmj.2018.0259.
- Bissinger A. Cardiac Autonomic Neuropathy: Why Should Cardiologists Care about That? J. Diabetes Res. 2017. 2017. 5374176. doi: 10.1155/2017/5374176.
- Duque A., Mediano M.F.F., De Lorenzo A., Rodrigues L.F. Jr. Cardiovascular autonomic neuropathy in diabetes: Pathophysiology, clinical assessment and implications. World J. Diabetes. 2021. 12(6). 855-867. doi: 10.4239/wjd.v12.i6.855.
- Agashe S., Petak S. Cardiac Autonomic Neuropathy in Diabetes Mellitus. Methodist Debakey Cardiovasc J. 2018. 14(4). 251-256. doi: 10.14797/mdcj-14-4-251.
- Osailan A. Cardiovascular autonomic neuropathy in people with type 2 diabetes mellitus; investigation of its association with classical cardiovascular risk factors using cardiovascular autonomic reflex tests: a cross-sectional study. Egypt Heart J. 2021. 73(1). 44. doi: 10.1186/s43044-021-00168-3.
- Mala S., Potockova V., Hoskovcova L., Pithova P., Brabec M., Kulhankova J., Keil R., Riedlbauchova L., Broz J. Cardiac autonomic neuropathy may play a role in pathogenesis of atherosclerosis in type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 2017. 134. 139-144. doi: 10.1016/j.diabres.2017.10.002.
- Huang C.C., Lee J.J., Lin T.K., Tsai N.W., Huang C.R., Chen S.F., Lu C.H., Liu R.T. Diabetic Retinopathy Is Strongly Predictive of Cardiovascular Autonomic Neuropathy in Type 2 Diabetes. J. Diabetes Res. 2016. 2016. 6090749. doi: 10.1155/2016/6090749.
- Bilal N., Erdogan M., Ozbek M., Cetinkalp S., Karadeniz M., Ozgen A.G., Saygili F., et al. Increasing severity of cardiac autonomic neuropathy is associated with increasing prevalence of nephropathy, retinopathy, and peripheral neuropathy in Turkish type 2 diabetics. J. Diabetes Complications. 2008. 22(3). 181-5. doi: 10.1016/j.jdiacomp.2006.12.003.
- Fong D.S., Warram J.H., Aiello L.M., Rand L.I., Krolewski A.S. Cardiovascular autonomic neuropathy and proliferative diabetic retinopathy. Am. J. Ophthalmol. 1995. 120(3). 317-21. doi: 10.1016/s0002-9394(14)72161-0.
- Zander E., Heinke P., Herfurth S., Reindel J., Ostermann F.E., Kerner W. Relations between diabetic retinopathy and cardiovascular neuropathy — a cross-sectional study in IDDM and NIDDM patients. Exp. Clin. Endocrinol. Diabetes. 1997. 105(6). 319-26. doi: 10.1055/s-0029-1211772.
- Kaze A.D., Yuyun M.F., Ahima R.S., Sachdeva M.M., Echouffo-Tcheugui J.B. Association of Heart Rate Variability with Progression of Retinopathy among Adults with Type 2 Diabetes. Diabetic Medicine. First published: 25 April 2022. https://doi.org/10.1111/dme.14857.
- De Barros J.A., Macartney M.J., Peoples G.E., Notley S.R., Herry C.L., Kenny G.P. The impact of age, type 2 diabetes and hypertension on heart rate variability during rest and exercise at increasing levels of heat stress. Eur. J. Appl. Physiol. 2022. 122(5). 1249-1259. doi: 10.1007/s00421-022-04916-4.