Международный эндокринологический журнал Том 18, №4, 2022
Вернуться к номеру
The role of vitamin D for the management of depression in patients with autoimmune thyroiditis and hypothyroidism in the West-Ukrainian population
Авторы: Iryna Kamyshna
I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
Актуальність. Відомо, що тиреоїдит Хашимото є одним із найчастіших ендокринних захворювань, яке вражає населення різних вікових груп і може призвести до гіпотиреозу. Ця патологія також є одним із найбільш поширених автоімунних захворювань. Пацієнти з гіпотиреозом часто відчувають ознаки депресії, яка переважає серед інших симптомів гіпотиреозу. Дані останніх досліджень довели, що дефіцит вітаміну D може викликати прояви депресії в населення. Метою проведеної роботи є вивчення впливу холекальциферолу в пацієнтів з автоімунним тиреоїдитом та гіпотиреозом у західноукраїнській популяції на рівень депресії у цих хворих. Матеріали та методи. У дослідження були включені 56 пацієнтів із гіпотиреозом, спричиненим автоімунним тиреоїдитом. Визначали наявність депресії за допомогою шкали депресії Гамільтона (HDRS). Обстеження проводили на початку та в кінці 12-тижневого лікування. Результати. У пацієнтів першої групи, які отримували холекальциферол і L-тироксин, рівень депресії за шкалою Гамільтона знизився на 40 %, тоді як у пацієнтів, які отримували тільки L-тироксин, рівень депресії знизився на 25 %. Крім того, спостерігалася вірогідна різниця між пацієнтами першої та другої груп після лікування (р = 0,003). При аналізі ефекту лікування в пацієнтів першої групи з додатковим призначенням холекальциферолу на тлі L-тироксину у 21,4 % пацієнтів зникли прояви депресії. Крім того, спостерігалося зниження рівня депресії у решти пацієнтів цієї групи. Ступінь вираженості депресивних проявів зменшився від середньої тяжкості до легкого депресивного розладу. Висновки. Пацієнтам, які страждають на автоімунний тиреоїдит та гіпотиреоз, слід призначати вітамін D, що сприяє зменшенню в них частоти депресивних розладів.
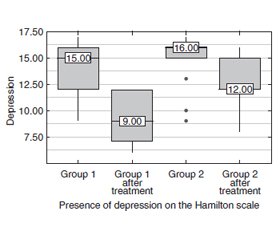
Background. Hashimoto’s thyroiditis is known to be an essential endocrine disease that affects the population and may lead to hypothyroidism. This disease is one of the most commonly spread autoimmune diseases. Hypothyroid patients frequently experience features of depression, which is prevalent among other symptoms in hypothyroidism. Data from recent research has proved that vitamin D deficiency may cause depression manifestations in the population. The purpose of the work is to study the effect of cholecalciferol in patients with autoimmune thyroiditis and hypothyroidism in the West-Ukrainian population on the level of depression in these patients. Materials and methods. The study included the 56 patients with hypothyroidism (H) caused by autoimmune thyroiditis (AIT). We identified the severity of depression levels using the Hamilton Depression Rating Scale (HDRS), which is reliable for depression assessment. Examinations were performed at the beginning and end of the 12-week treatment. Results. In patients of group 1 who received cholecalciferol and L-thyroxine, the level of depression on the Hamilton scale decreased by 40 %, while in patients who received only L-thyroxine, the level of depression decreased by 25 %. In addition, there was a significant difference between patients in groups 1 and 2 after treatment (p = 0.003). That is, treatment with additional cholecalciferol on the background of L-thyroxine was more effective than treatment with L-thyroxine alone. Analyzing the effect of treatment in patients with Group 1 with the additional appointment of cholecalciferol on the background of L-thyroxine in 21.4 % of patients disappeared depression. In addition, there was a decrease in depression in other patients in this group. Thus, in the remaining patients the severity of depressive manifestations decreased from moderate severity to mild depressive disorder. At the same time, after treatment only L-thyroxine depressive disorder of moderate severity decreased from 78.6 to 35.7 % to mild depressive disorder, but complete disappearance of depression in this group of patients after treatment was not observed. Conclusions. Vitamin D supplementation should be administered in patients suffering from autoimmune thyroiditis and hypothyroidism which may correct depression disorders in these patients.
автоімунний тиреоїдит; гіпотиреоз; депресія
autoimmune thyroiditis; hypothyroidism; depression
Introduction
Materials and methods
Results
/12_2.jpg)
Discussion
Conclusions
- Caturegli P., De Remigis A., Rose N.R. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun. Rev. 2014. 13(4-5). 391-7. doi: 10.1016/j.autrev.2014.01.007.
- Gorkhali B., Sharma S., Amatya M., Acharya D., Sharma M. Anxiety and Depression among Patients with Thyroid Function Disorders. J. Nepal Health Res. Counc. 2020. 18(3). 373-378. doi: 10.33314/jnhrc.v18i3.2499. PMID: 33210626.
- Ittermann T., Völzke H., Baumeister S.E., Appel K., Grabe H.J. Diagnosed thyroid disorders are associated with depression and anxiety. Soc. Psychiatry Psychiatr. Epidemiol. 2015. 50(9). 1417-25. doi: 10.1007/s00127-015-1043-0.
- Krysiak R., Kowalcze K., Okopień B. The impact of exogenous vitamin D on thyroid autoimmunity in euthyroid men with autoimmune thyroiditis and early-onset androgenic alopecia. Pharmacol. Rep. 2021. doi: 10.1007/s43440-021-00295-3.
- Erensoy H. The association between anxiety and depression with 25(OH)D and thyroid stimulating hormone levels. Neurosciences (Riyadh). 2019. 24(4). 290-295. doi: 10.17712/nsj.2019.4.20190028.
- Casseb G.A.S., Kaster M.Р., Rodrigues A.L.S. Potential Role of Vitamin D for the Management of Depression and Anxiety. CNS Drugs. 2019. 33(7). 619-637. doi: 10.1007/s40263-019-00640-4.
- Garber J.R., Cobin R.H., Gharib H., Hennessey J.V., Klein I., Mechanick J.I. et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr. Pract. 2012. 18(6). 988-1028. doi: 10.4158/EP12280.GL.
- Blacker D. Rating Scales in Psychiatry. In: Sadock B.J., Sadock V.A., Eds. Comprehensive Textbook of Psychiatry (8th). Lippincott Williams & Wilkins, Philadelphia. 2007. Р. 929-943.
- Leyhe T., Müssig K. Cognitive and affective dysfunctions in autoimmune thyroiditis. Brain Behav. Immun. 2014. 41. 261-6. doi: 10.1016/j.bbi.2014.03.008.
- Siegmann E.М., Müller H.Н.О., Luecke C., Philipsen A., Kornhuber J., Grömer T.W. Association of Depression and Anxiety Disorders With Autoimmune Thyroiditis: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2018. 75(6). 577-584. doi: 10.1001/jamapsychiatry.2018.0190.
- Holick M.F., Chen T.C. Vitamin D deficiency: a worldwide problem with health consequences. Am. J. Clin. Nutr. 2008. 87(4). 1080S-6S. doi: 10.1093/ajcn/87.4.1080S.
- Wang S., Liu Y., Zhao N., Cui X., Huang M., Li Y., Shan Z., Teng W. IL-34 Expression Is Reduced in Hashimoto’s Thyroiditis and Associated With Thyrocyte Apoptosis. Front. Endocrinol. (Lausanne). 2018. 9. 629. doi: 10.3389/fendo.2018.00629.
- Kust D., Matesa N. The impact of familial predisposition on the development of Hashimoto’s thyroiditis. Acta Clin. Belg. 2020. 75(2). 104-108. doi: 10.1080/17843286.2018.1555115.
- Cai Y.J., Wang F., Chen Z.Х., Li L., Fan H., Wu Z.В. et al. Hashimoto’s thyroiditis induces neuroinflammation and emotional alterations in euthyroid mice. J. Neuroinflammation. 2018. 15(1). 299. doi: 10.1186/s12974-018-1341-z.
- Roehlen N., Doering C., Hansmann M.L., Gruenwald F., Vorlaender C., Bechstein W.O. et al. Vitamin D, FOXO3a, and Sirtuin1 in Hashimoto’s Thyroiditis and Differentiated Thyroid Cancer. Front. Endocrinol. (Lausanne). 2018. 9. 527. doi: 10.3389/fendo.2018.00527.
- Häusler D., Weber M.S. Vitamin D Supplementation in Central Nervous System Demyelinating Disease-Enough Is Enough. Int. J. Mol. Sci. 2019. 20(1). 218. doi: 10.3390/ijms20010218.
- Kalueff A.V., Tuohimaa P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr. Opin. Clin. Nutr. Metab. Care. 2007. 10(1). 12-9. doi: 10.1097/MCO.0b013e328010ca18.
- Bertone-Johnson E.R. Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr. Rev. 2009. 67(8). 481-92. doi: 10.1111/j.1753-4887.2009.00220.x.
- Patrick R.Р., Ames B.N. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J. 2014. 28(6). 2398-413. doi: 10.1096/fj.13-246546.
- Pertile R.А.N., Cui X., Hammond L., Eyles D.W. Vitamin D regulation of GDNF/Ret signaling in dopaminergic neurons. FASEB J. 2018. 32(2). 819-828. doi: 10.1096/fj.201700713R.
- Moradi H., Sohrabi M., Taheri H., Khodashenas E., Movahedi A. The effects of different combinations of perceptual-motor exercises, music, and vitamin D supplementation on the nerve growth factor in children with high-functioning autism. Complement Ther. Clin. Pract. 2018. 31. 139-145. doi: 10.1016/j.ctcp.2018.02.005.
- Sepehrmanesh Z., Kolahdooz F., Abedi F., Mazroii N., Assarian A., Asemi Z., Esmaillzadeh A. Vitamin D Supplementation Affects the Beck Depression Inventory, Insulin Resistance, and Biomarkers of Oxidative Stress in Patients with Major Depressive Disorder: A Randomized, Controlled Clinical Trial. J. Nutr. 2016. 146(2). 243-8. doi: 10.3945/jn.115.218883.
- Cheng Y.С., Huang Y.С., Huang W.L. The effect of vitamin D supplement on negative emotions: A systematic review and meta-analysis. Depress. Anxiety. 2020. 37(6). 549-564. doi: 10.1002/da.23025.
- Lopez R.В., Denny B.Т., Fagundes C.P. Neural mechanisms of emotion regulation and their role in endocrine and immune functioning: A review with implications for treatment of affective disorders. Neurosci Biobehav. Rev. 2018. 95. 508-514. doi: 10.1016/j.neubiorev.2018.10.019.
- Kaminskyi O.V., Pankiv V.I., Pankiv I.V., Afanasyev D.E. Vitamin D content in population of radiologically contaminated areas in Chernivtsi oblast (pilot project). Probl. Radiac. Med. Radiobiol. 2018. 23. 442-451. English, Ukrainian. doi: 10.33145/2304-8336-2018-23-442-451.
- Sabir M.S., Haussler M.R., Mallick S., Kaneko I., Lucas D.А., Haussler C. et al. Optimal vitamin D spurs serotonin: 1,25-dihydroxyvitamin D represses serotonin reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines. Genes Nutr. 2018. 13. 19. doi: 10.1186/s12263-018-0605-7. PMID: 30008960. PMCID: PMC6042449.
- Moore M.Е., Piazza A., McCartney Y., Lynch M.A. Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Biochem. Soc. Trans. 2005. 33(Pt 4). 573-7. doi: 10.1042/BST0330573.

/11.jpg)
/12.jpg)