Международный эндокринологический журнал Том 18, №4, 2022
Вернуться к номеру
Морфологічні особливості доброякісних вогнищевих новоутворень щитоподібної залози при хворобі Грейвса
Авторы: Buldygina Yu.V. (1), Zelinskaya A.V. (1), Zurnadzhy L.Yu. (1), Tarashchenko Yu.M. (1), Shlyakhtych S.L. (2), Tronko M.D. (1)
(1) — State Institution “V.P. Komisarenko Institute of Endocrinology and Metabolism of the National Academy of Medical Sciences of Ukraine”, Kyiv, Ukraine
(2) — 3rd Kyiv City Clinical Hospital, Kyiv, Ukraine
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
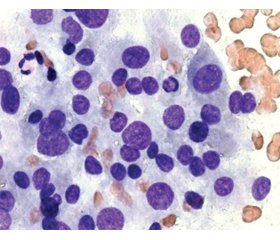
Актуальність. Морфологічні зміни щитоподібної залози при хворобі Грейвса (ХГ) досить різноманітні: у більшості випадків спостерігається дифузне збільшення щитоподібної залози (дифузний зоб), в окремих випадках залоза не збільшена, а в деяких пацієнтів наявні вогнищеві поодинокі й множинні новоутворення, включно з раком щитоподібної залози. За даними деяких досліджень, у пацієнтів із ХГ вогнищеві утворення спостерігаються в 10–31 % випадків, а в половини хворих вони виникають на тлі лікування тіонамідами. Метою даної роботи було ретроспективне вивчення морфологічних особливостей доброякісних новоутворень щитоподібної залози внаслідок ХГ за результатами цитологічних і патоморфологічних досліджень. Матеріали та методи. Дослідження проведено в клініці ДУ «Інститут ендокринології та обміну речовин імені В.П. Комісаренка НАМН України». Спочатку були відібрані всі пацієнти із ХГ, прооперовані у відділенні ендокринної хірургії з 2008 по 2019 р. (1854 пацієнти). Потім серед них були відібрані ті, у кого за даними патогістологічного дослідження були доброякісні вогнищеві утворення. Результати. Частота доброякісних утворень щитоподібної залози при ХГ становить 22,06 % у жінок віком від 26 до 55 років. Цитологічні дослідження пунктатів новоутворень щитоподібної залози при ХГ дозволили чітко встановити їх доброякісну природу (BSRTC-2) в 94,3 % випадків. У 5,7 % випадків були встановлені цитологічні категорії BSRTC-3 і BSRTC-4. Уникнути цитологічної гіпердіагностики, яка є характерною для доброякісних новоутворень щитоподібної залози на тлі ХГ, дозволяють позитивні імуноцитохімічні реакції до тиреоїдної пероксидази й тиреоглобуліну і відсутність цитокератину-17. Висновки. Серед патогістологічно підтверджених доброякісних новоутворень щитоподібної залози при ХГ у 63,82 % випадків гістологічним діагнозом був аденоматозний або колоїдний зоб, у 34,47 % відзначалися ознаки вузлової гіперплазії, у 1,71 % — наявність тиреоїдиту.
Background. The morphology of the thyroid in Graves’ disease (GD) can be quite diverse: in most cases there is a diffuse enlargement of the thyroid gland (diffuse goiter), in some cases it is not increased, and in some patients there are focal neoplasms (single and multiple), including thyroid cancer. According to some studies, in patients with Graves’ disease, focal formations are observed in 10–31% of cases, and in half of the patients, they appear against the background of treatment with thionamides. The purpose of this paper was a retrospective study of the morphological features of benign thyroid neoplasms due to GD based on the results of cytological and pathomorphological studies. Materials and methods. The study was performed at the clinic of the State Institution “V.P. Komisarenko Institute of Endocrinology and Metabolism of the National Academy of Medical Sciences of Ukraine”. All patients with GD who were operated in the Department of Endocrine Surgery from 2008 to 2019 (1854 patients) were first selected, and then those who had benign focal formations according to the pathohistological examination were selected among them. Results. The frequency of benign thyroid neoplasms in GD is 22.06 % in women aged 26 to 55 years. Cytological studies of thyroid neoplasm punctures in GD made it possible to clearly establish their benign nature (BSRTC-2) in 94.3 % of cases. In 5.7 % of cases, cytological categories BSRTC-3 and BSRTC-4 were established. Positive immunocytochemical reactions to thyroid peroxidase and thyroglobulin, and the absence of CK17 allow avoiding cytological overdiagnosis, characteristic of benign neoplasms of the thyroid gland in GD. Conclusions. Among pathohistologically confirmed benign neoplasms of the thyroid gland in GD, in 63.82 % of cases a histological diagnosis was adenomatous or colloid goiter, in 34.47 % — signs of nodular hyperplasia, in 1.71 % — the presence of thyroiditis.
хвороба Грейвса; щитоподібна залоза; доброякісні новоутворення щитоподібної залози
Graves’ disease; thyroid gland; benign thyroid neoplasms
Introduction
Materials and methods
Results
/17_2.jpg)
/18_2.jpg)
Discussion
Conclusions
- Ehlers M., Schott M., Allelein S. Graves’ disease in clinical perspective. Front Biosci (Landmark Ed). 2019. 24(1). 35-47. doi: 10.2741/4708.
- Wiersinga W.M. Clinical Relevance of Environmental Factors in the Pathogenesis of Autoimmune Thyroid Disease. Endocrinol. Metab. (Seoul). 2016. 31(2). 213-22. doi: 10.3803/EnM.2016.31.2.213.
- Antonelli A., Ferrari S.M., Ragusa F., Elia G., Paparo S.R., Ruffilli I., Patrizio A. et al. Graves’ disease: Epidemiology, genetic and environmental risk factors and viruses. Best Pract. Res. Clin. Endocrinol. Metab. 2020. 34(1). 101387. doi: 10.1016/j.beem.2020.101387.
- Rapoport B., Aliesky H.A., Chen C.R., McLachlan SM. Evidence that TSH Receptor A-Subunit Multimers, Not Monomers, Drive Antibody Affinity Maturation in Graves’ Disease. J. Clin. Endocrinol. Metab. 2015;100(6):E871-5. doi: 10.1210/jc.2015-1528.
- Bartalena L., Burch H.B., Burman K.D., Kahaly G.J. A 2013 European survey of clinical practice patterns in the management of Graves’ disease. Clin. Endocrinol. (Oxf.). 2016. 84(1). 115-20. doi: 10.1111/cen.12688.
- Lima P.C., Moura Neto A., Tambascia M.A., Zantut Wittmann D.E. Risk factors associated with benign and malignant thyroid nodules in autoimmune thyroid diseases. ISRN Endocrinol. 2013. 2013. 673146. doi: 10.1155/2013/673146.
- Staniforth J.U.L., Erdirimanne S., Eslick G.D. Thyroid carcinoma in Graves’ disease: A meta-analysis. Int. J. Surg. 2016. 27. 118-125. doi: 10.1016/j.ijsu.2015.11.027.
- Casella C., Morandi R., Verrengia A., Galani A., Molfino S., Cuka D., Groppo G., Cappelli C., Portolani N. Thyroid Cancer and Nodules in Graves’ Disease: A Single Center Experience. Endocr. Metab. Immune Disord. Drug Targets. 2021. 21(11). 2028-2034. doi: 10.2174/1871530321666201230111911.
- Papanastasiou A., Sapalidis K., Goulis D.G., Michalopoulos N., Mareti E., Mantalovas S., Kesisoglou I. Thyroid nodules as a risk factor for thyroid cancer in patients with Graves’ disease: A systematic review and meta-analysis of observational studies in surgically treated patients. Clin. Endocrinol. (Oxf.). 2019. 91(4). 571-577. doi: 10.1111/cen.14069.
- Khan S.H., Rather T.A., Makhdoomi R., Malik D. Nodular Graves’ disease with medullary thyroid cancer. Indian J. Nucl. Med. 2015. 30(4). 341-4. doi: 10.4103/0972-3919.164022.
- Kahaly G.J. Management of Graves Thyroidal and Extrathyroidal Disease: An Update. J. Clin. Endocrinol. Metab. 2020. 105(12). 3704-20. doi: 10.1210/clinem/dgaa646.
- Болгов М.Ю. Автоматизация медицинских систем. Киев: Издательство Куприянова, 2006. 464 с.
- Pathology and Genetics of Tumours of Endocrine Organs. WHO Classification of Tumours. 3rd Edition. 2004. Vol. 8. Edited by DeLellis R.A., Lloyd R.V., Heitz P.U., Eng C. IARC Press, Lyon, France.
- Зелінська Г.В. Цитохімічне та імуноцитохімічне дослідження експресії тиреоїдної пероксидази в передопераційній діагностиці папілярного раку щитоподібної залози та його радіойодрезистентних метастазів. Онкологія. 2019. 21(3). 1-6. doi: 10.32471/oncology.2663-7928.t-21-3-2019-g.7925.
- Зелінська Г.В. Цитокератин 17 і тиреоїдна пероксидаза в якості імуноцитохімічних маркерів доопераційного прогнозування радіойодрезистентності та ефективності радіойодтерапії папілярного раку щитоподібної залози. Онкологія. 2019. 21(1). 31-35. doi: 10.32471/oncology.2663-7928.t-21-3-2019-g.7376.
- Anderson S.R., Mandel S., LiVolsi V.A., Gupta P.K., Baloch Z.W. Can cytomorphology differentiate between benign nodules and tumors arising in Graves’ disease? Diagn. Cytopathol. 2004. 31(1). 64-7. doi: 10.1002/dc.20075.
- Hang J.F., Lilo M.T., Bishop J.A., Ali S.Z. Diagnostic Accuracy of Fine Needle Aspiration in Thyroid Nodules Arising in Patients with Graves Disease. Acta Cytol. 2017. 61(2). 117-124. doi: 10.1159/000464094.

/17.jpg)
/17_3.jpg)
/18_3.jpg)
/18.jpg)