Международный эндокринологический журнал Том 18, №4, 2022
Вернуться к номеру
Інсулінорезистентність: метаболічні і соматичні зміни у дітей
Авторы: Gromnatska N.M., Sklyarova O.Y., Kulya O.O.
Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
Актуальність. Інсулінорезистентність є базовим фактором етіології та патогенезу цукрового діабету 2-го типу та метаболічного синдрому і може передувати їм за багато років. Раннє виявлення початку інсулінорезистентності та пов’язаних з нею метаболічних факторів ризику у дітей запобігає розвитку цукрового діабету в дорослому житті. Мета: виявити метаболічні і соматичні зміни у дітей з інсулінорезистентністю. Матеріали та методи. З 182 дітей загальної вибірки, у яких визначено рівень базального інсуліну і глюкози, HOMA-IR та індексу глюкоза/інсулін сформовано дві групи: група 1 — 56 (30,8 %) дітей з інсулінорезистеністю, група 2 — 126 (69,2 %) дітей з нормальною чутливістю до інсуліну. Дітям проведено антропометрію, ліпідограму (загальний холестерин, тригліцериди, ХС ЛПВЩ, ХС ЛПНЩ, ХС ЛПДНЩ), лептин. Результати. З обстеженої когорти 56 дітей мали генералізоване ожиріння (ІМТ > 95-го перцентиля), 71 дитина — абдомінальне ожиріння (окружність талії > 90-го перцентиля), 55 дітей — нормальну масу тіла (ІМТ < 90-го перцентиля). Інсулінорезистентність виявлена у 21 (37,5 %) дитини з генералізованим ожирінням, 38 (39,4 %; p = 0,049) дітей з абдомінальним ожирінням і 7 (12,7 %) дітей з нормальним ІМТ (p = 0,003). У інсулінорезистентних дітей ІМТ, окружність талії і стегон були більшими, ніж в інсуліночутливих дітей. Ліпідний профіль у дітей з різною чутливістю до інсуліну не відрізнявся, проте встановлена висока кореляційна залежність ранішньої глюкози з індексом тригліцериди/ХС ЛПВЩ (r = 0,53; p = 0,001), базального інсуліну з тригліцеридами (r = 0,34; p = 0,018) та індексом тригліцериди/ХС ЛПВЩ (r = 0,54; p = 0,001). HOMA-IR корелював з ХС ЛПДНЩ (r = 0,40; p = 0,005), тригліцеридами (r = 0,49; p = 0,001), індексом тригліцериди/ХС ЛПВЩ (r = 0,43; p = 0,002). Індекс глюкоза/інсулін перебував у тісній залежності з індексом тригліцериди/ХС ЛПВЩ. Частота діагностики артеріальної гіпертензії у дітей з інсулінорезистеністю на 33,8 % перебільшувала аналогічний показник у інсуліночутливих дітей. Рівень сироваткового лептину у дітей з інсулінорезистентністю був в 1,8 раза вищим. Висновки. Інсулінорезистентність пов’язана з кардіометаболічними факторами ризику, такими як генералізоване та абдомінальне ожиріння, гіпертензія, дисліпідемія, гіперлептинемія і лептинорезистентність, і є біомаркером скринінгу кардіометаболічних захворювань.
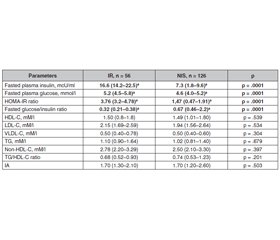
Background. Insulin resistance is the major sign of etiology and pathogenesis of type 2 diabetes mellitus and metabolic syndrome and can precede its development for many years. Early identifying the beginning of insulin resistance in children is important to prevent diabetes mellitus in adult life. The purpose was to identify metabolic and somatic changes in children with insulin resistance. Material and methods. Out of 182 children of the general sample, who was estimated fasting plasma insulin and glucose, HOMA-IR, and glucose/insulin ratio, 2 groups were formed: group 1 — children with IR — 56 (30.8 %) and group 2 — 126 (69.2 %) children with normal insulin sensitivity. In children anthropometric data, lipid metabolism (total cholesterol, triglycerides, HDL-C, LDL-C, VLDL-C), blood pressure, leptin were determined. Results. From examined subjects 56 children were generally obese (BMI > 95th percentile), 71 children were abdominally obese (WC > 90th percentile), 55 children were with normal body mass (BMI < 90th percentile). Insulin resistance was identified in 21 (37.5 %) children with general obesity more rarely, than in 38 (39.4 %) children with abdominal obesity (p = .049) and in 7 (12.7 %) children with normal BMI (p = .003). In insulin-resistant children BMI, waist and hip circumference was larger than in children with normal insulin sensitivity. The lipid profile in children with different insulin sensitivity did not differ, but in insulin-resistant children an association of basal glucose with TG/HDL-C ratio (r = .53; p = .001), blood insulin with TG (r = .34; p = .018), and TG/HDL-C ratio (r = .54; p = .001) was estimated. The HOMA-IR significantly correlated with VLD-C (r = .40; p = .005), TG (r = .49; p = .001), TG/HDL-C ratio (r = .43; p = .002). The glucose/insulin ratio was in significant association with the TG/non-HDL-C ratio. The incidence of hypetension (> 95th percentile) diagnosis in insulin-resistant children was by 33.8 % higher (p = .001). Blood leptin concentration was 1.8 falled higher in insulin-resistant children and significantly correlates with waist circumference, fasting insulin, HOMA-IR, and diastolic blood pressure. Conclusions. Insulin resistance is related to cardiometabolic risks, such as general and abdominal obesity, hypertension, dyslipidemia, hyperleptinemia, and leptin resistance, and is a screening biomarker for children and adolescents with an increased risk of cardiometabolic diseases.
інсулінорезистентність; метаболічні фактори ризику; діти
insulin resistance; metabolic risk factors; children
Introduction
Materials and methods
Results
Discussion
Conclusions
- Rehman K., Akash M.S.H. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J. Biomed. Science. 2016. 23. 87. DOI. 10.1186/s12929-0303-y.
- Martín-Timón I., Sevillano-Collantes C., Segura-Galindo A., Del Cañizo-Gómez F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes. 2014. 5(4). 444-70. doi: 10.4239/wjd.v5.i4.444.
- Gayoso-Diz P., Otero-González A., Rodriguez-Alvarez M.X., et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013. 13. 47. DOI. 10.1186/1472-6823-13-47.
- Çin N.N.A., Yardımcı H., Koç N., et al. Triglycerides/high-density lipoprotein cholesterol is a predictor similar to the triglyceride-glucose index for the diagnosis of metabolic syndrome using International Diabetes Federation criteria of insulin resistance in obese adolescents: a cross-sectional study. J. Pediat. Endocrin. Metab. 2020. Published online. DOI: https://doi.org/10.1515/jpem-2019-0310.
- Sorokman T., Makarova O. Somatic and mental health of obese children. International Journal of Endocrinology (Ukraine). 2020. 16(7). 543-550. https://doi.org/10.22141/2224-0721.16.7.2020.219008.
- Bolshova O., Malinovska T. The Content of Ghrelin and Leptin in the Blood Plasma of Children and Adolescents With Hypothalamic Dysfunction. International Journal of Endocrinology (Ukraine). 2018. 14 (8). 719-24. doi:10.22141/2224-0721.14.8.2018.154849.
- Falalyeyeva T., Mamula Y., Scarpellini E., Leshchenko I., Humeniuk A., Pankiv I., Kobyliak N. Probiotics and obesity associated disease: an extended view beyond traditional strains. Minerva Gastroenterol. (Torino). 2021. 67(4). 348-356. doi: 10.23736/S2724-5985.21.02909-0.
- Castorani V., Polidori N., Giannini C., Blasetti A., Chiarelli F. Insulin resistance and type 2 diabetes in children. Ann. Pediatr. Endocrinol. Metab. 2020. 25(4). 217-226. doi: 10.6065/apem.2040090.045.
- Manios Y., Moschonis G., Kourlaba G., et al. Prevalence and independent predictors of insulin resistance in children from Crete, Greece: the Children Study. Diabet. Med. 2008. 25(1). 65-72. doi: 10.1111/j.1464-5491.2007.02318.
- Grant A.M., Taungapeau F.K., McAuley K.A., et al. Body mass index status is effective in identifying metabolic syndrome components and insulin resistance in Pacific Island teenagers living in New Zeeland. Metabolism: Clin. Experim. 2008. 57(4). 511-516. doi: 10.1016/j.metabol.2007.11.13.
- Hirscler V., Maccalini G., Karam C., et al. Age girls are insulin resistant than boys? Clin. Biochem. 2009. 42(10-11). 1051-6. doi: 10.1016/j.clinbiochem.2009.03.002.
- Gromnatska N., Cherkas A., Lemishko B., Kulya O. The pattern of metabolic syndrome in children with abdominal obesity. Georgian Med. News. 2019. 289. 68-72. PMID: 31215882.
- Petersen M.C., Shulman G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018. 98(4). 2133-2223. doi: 10.1152/physrev.00063.2017.
- Czech M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017. 23(7). 804-814. doi: 10.1038/nm.4350.
- Pankiv V.I., Yuzvenko T.Yu., Pashkovska N.V., Pankiv I.V. Effect of vitamin D on insulin resistance and anthropometric parameters in patients with type 2 diabetes mellitus. Clinical Endocrinology and Endocrine Surgery. 2019. 1(65). 53-58. DOI: http://doi.org/10.30978/CEES-2019-1-53.
- Soriguer F., Gutiérrez-Repiso C., Rubio-Martín E., García-Fuentes E., et al. Metabolically healthy but obese, a matter of time? Findings from the prospective Pizarra study. J. Clin. Endocrinol. Metab. 2013. 98(6). 2318-25. doi: 10.1210/jc.2012-4253.
- Gepstein V., Weiss R. Obesity as the Main Risk Factor for Metabolic Syndrome in Children. Front Endocrinol. (Lausanne). 2019. 10. 568. doi: 10.3389/fendo.2019.00568.
- Pankiv I.V. Intercommunication between serum vitamin D concentration and the body mass index. Obesity Reviews. 2020. 21 (Suppl. 1). EP 411. https://onlinelibrary.wiley.com/doi/epdf/10.1111/obr.13118.
- da Silva C.C., Zambon M.P., Vasques A.C.J., Camilo D.F., et al. Brazilian Metabolic Syndrome Study (BRAMS) Investigators. Homeostatic model assessment of adiponectin (HOMA-Adiponectin) as a surrogate measure of insulin resistance in adolescents: Comparison with the hyperglycaemic clamp and homeostatic model assessment of insulin resistance. PLoS One. 2019. 14(3). e0214081. doi: 10.1371/journal.pone.0214081.
- Zabarsky G., Beek C., Hagman E., Pierpont B., Caprio S., Weiss R. Impact of severe obesity on cardiovascular risk factors in youth. J. Pediatr. 2018. 192. 105-14. Doi: 10.1016/j.jpeds.2017.09.066.
- Hershkop K., Besor O., Santoro N., Pierpont B., Caprio S., Weiss R. Adipose insulin resistance in obese adolescents across the spectrum of glucose tolerance. J. Clin. Endocrinol. Metab. 2016. 101. 2423-31. Doi: 10.1210/jc.2016-1376.
- Ahila A., Vimalraj V., Raghupathy P. Insulin resistance and adverse lipid profile in obese prepubertal South Indian children. Intern. J. Pediat. Endocrinol. 2015. 78. https://doi.org/10.1186/1687-9856-2015-81-P78.
- van der Aa M.P., Fazeli Farsani S., Knibbe C.A., de Boer A., van der Vorst M.M. Population-Based Studies on the Epidemiology of Insulin Resistance in Children. J. Diabetes Res. 2015. 2015. 362375. doi: 10.1155/2015/362375.
- Takahashi T., Okamoto T., Sato Y., Hayashi A., Ueda Y., Ariga T. Glucose metabolism disorders in children with refractory nephrotic syndrome. Pediatr. Nephrol. 2020. 35(4). 649-657. doi: 10.1007/s00467-019-04360-1.
- Mastrangelo A., Martos-Moreno G.Á., García A., Barrios V., Rupérez F.J., Chowen J.A., Barbas C., Argente J. Insulin resistance in prepubertal obese children correlates with sex-dependent early onset metabolomic alterations. Int. J. Obes (Lond). 2016. 40(10). 1494-1502. doi: 10.1038/ijo.2016.92.
- Armstrong M.J., Hazlehurst J.M., Hull D., Guo K., Borrows S., Yu J., Gough S.C., Newsome P.N., Tomlinson J.W. Abdominal subcutaneous adipose tissue insulin resistance and lipolysis in patients with non-alcoholic steatohepatitis. Diabetes Obes. Metab. 2014. 16(7). 651-60. doi: 10.1111/dom.12272.
- Abbasi F., Kohli P., Reaven G.M., Knowles J.W. Hypertriglyceridemia: A simple approach to identify insulin resistance and enhanced cardio-metabolic risk in patients with prediabetes. Diabetes Res. Clin. Pract. 2016. 120. 156-61. doi: 10.1016/j.diabres.2016.07.024.
- Chmyr N.V., Dutka R.J. Pathogenetic and clinical features of the metabolic syndrome associated with obesity and their prognostic value. Bukovinian Med. Bullet. 2017. 23(3). 10-15. DOI: 10.24061/2413-0737/XXI.2.82.1.2017.28. (in Ukrainian)
- Mitsuhashi T., Hibi K., Kosuge M., et al. Relation between hyperinsulinemia and nonculprit plaque characteristics in nondiabetic patients with acute coronary syndromes. JACC Cardiov Imaging. 2011. 4(4). 392-401. DOI: 10.1016/j.jcmg.2011.02.004.
- Gao F., Lucke-Wold B.P., Li X., Logsdon A.F., Xu L.C., Xu S., LaPenna K.B., et al. Reduction of Endothelial Nitric Oxide Increases the Adhesiveness of Constitutive Endothelial Membrane ICAM-1 through Src-Mediated Phosphorylation. Front Physiol. 2018. 8. 1124. doi: 10.3389/fphys.2017.01124.
- Boucher J., Kleinridders A., Kahn C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014. 6(1). a009191. doi: 10.1101/cshperspect.a009191.
- Janus A., Szahidewicz-Krupska E., Mazur G., Doroszko A. Insulin Resistance and Endothelial Dysfunction Constitute a Common Therapeutic Target in Cardiometabolic Disorders. Mediators Inflamm. 2016. 2016. 3634948. doi: 10.1155/2016/3634948.
- Ibarra-Reynoso L.R., Pisarchyk L., Pérez-Luque E.L., Garay-Sevilla M.E., Malacara J.M. Whole-Body and Hepatic Insulin Resistance in Obese Children. PLoS ONE. 2014. 9(11). :e113576. https://doi.org/10.1371/journal.pone.0113576.
- Moonishaa T.M., Nanda S.K., Shamraj M., Sivaa R., Sivakumar P., Ravichandran K. Evaluation of Leptin as a Marker of Insulin Resistance in Type 2 Diabetes Mellitus. Int. J. Appl Basic Med. Res. 2017. 7(3). 176-180. doi: 10.4103/ijabmr.IJABMR_278_16.
- Das P., Bhattacharjee D., Bandyopadhyay S.K., Bhattacharya G., Singh R. Association of obesity and leptin with insulin resistance in type 2 diabetes mellitus in Indian population. Indian. J. Physiol. Pharmacol. 2013. 57(1). 45-50. PMID: 24020098.
- Tibaldi J. Preserving insulin secretion in Type 2 diabetes mellitus. Expert Rev. Endocrinol. Metab. 2008. 3(2). 147-159. doi: 10.1586/17446651.3.2.147.
- Niswender K.D., Magnuson M.A. Obesity and the beta cell: lessons from leptin. J. Clin. Invest. 2007. 117(10). 2753-6. DOI: 10.1172/JCI33528.
- Mohiti J., Afkhami M., Babaei A. Relation between leptin and insulin in patients with type II diabetes mellitus. Int. J. Endocrinol. Metab. 2005. 3: 121-5.

/23.jpg)
/24.jpg)