Introduction
Pontine stroke is the most common ischemic stroke of the brainstem [1–3]. Isolated pontine infarctions are classified as either paramedian or lacunar pontine infarctions (LPI) [4–6]. It is widely accepted that paramedian pontine infarction is caused by the occlusion of basilar perforating branches, whereas LPI is caused by small vessel disease [7].
Clinical presentation of pontine stroke ranges from the classical crossed syndromes (such as Millard-Gubler, Foville, and Raymond-Cestan) to the less common pure motor, pure sensory stroke, or respiratory and cardiac dysfunction [8–12]. Early diagnosis and adequate understanding of the clinical presentation of LPI are essential for evaluating and managing the disease [13–15].
The purpose: to determine clinical and imaging features of lacunar pontine infarction at an early stage in a prospective hospital-based cohort study.
Materials and methods
The study settings, patterns, definitions, inclusion, and exclusion criteria have been reported in detail previously [16–24]. Briefly, 120 patients with MRI-positive acute posterior circulation stroke were consecutively selected. All of them were admitted to the Neurological center of the University Hospital, Oleksandrivska Clinical Hospital, between 2011 and 2020. The Hospital represents the largest tertiary care center in the capital of Ukraine, Kyiv.
Results
Among 120 consecutively selected patients with MRI-positive acute posterior circulation stroke, 38 were diagnosed with pontine infarctions. Of them, 15 patients, admitted within 6 hours after onset, were diagnosed with LPI and formed a study group. All 15 MRI-positive patients with LPI had a history of hypertension, and 40.0 % of them had diabetes. All LPI were isolated.
Most often, in 8 (53.3 %) cases, a pure motor LPI was detected, caused by damage to the pyramidal pathways in the area of the base of the pons. Pronounced hemiparesis in the acute period of a stroke was observed in one patient, moderate in two, and mild in five. Equal by the strength, weakness in the arm and leg was presented in three cases; more pronounced weakness in the arm was detected in five patients. Dysarthria was moderate in three cases and mild in two, central facial palsy was found in two patients. Pure motor hemiparesis was not accompanied by any impairment of speech, sensitivity, vision, brain stem function, hearing loss, tinnitus, diplopia, and gross nystagmus.
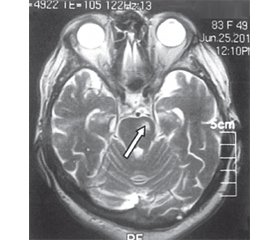
According to the MRI results, brain lesions in LPI patients were more often localized in the caudal part of the pons (n = 4). LPI was located in the middle part of the pons in two patients, and another two patients had LPI in the rostral part of the pons. All lesions were below 1 cm3. For illustration, we present brain MRI of patient K. (Fig. 1).
Pure sensory pontine strokes occurred in two (13.3 %) patients. In one person, a complete hemisensory syndrome was observed. It manifested by the loss of all types of sensitivity by the conductive type and was due to damage to the dorsal part of the pons. Another patient was diagnosed with incomplete hemisensory (cheiro-oral-pedal) syndrome in the presence of clinical symptoms of hypalgesia in the angle of the mouth, palm, and foot on one side without motor impairment.
Ataxic hemiparesis was diagnosed in four (26.7 %) patients. The typical localization of LPI lesion, in this case, was the rostral part of the pons, closer to the midline. The neurological status showed hemiataxia, moderate weakness of the leg, and slight paresis of the hand.
Dysarthria-clumsy hand pontine syndrome was detected in one (7 %) patient. The syndrome of dysarthria and clumsy hand was detected, which was accompanied by dysarthria and pronounced dysmetria of the arm and leg. The LPI lesion was localized in the paramedian area of the basal parts of the pons.
The neurological deficit in patients with LPI by the National Institutes of Health Stroke Scale (NIHSS) and
B. Hoffenberth scale was 6.3 ± 1.1 points and 8.7 ± 1.2 points, respectively. LPI patients’ reliable recovery according to the NIHSS occurred on the 7th day and by the scale of B. Hoffenberth, on the 14th day (0–1 points on the modified Rankin scale (mRS)).
Discussion
Lacunar stroke is an ischemic infarction of less than 15 mm in diameter located in the territory of the cerebral penetration arteriole [25–27]. Lacunar infarctions most commonly occur in the lenticular nucleus, thalamus, frontal lobe white matter, pons, basal ganglia, internal capsule, and caudate nucleus and are caused by occlusion of the deep perforating blood vessels [28]. Small vessel disease is most commonly associated with hypertension and diabetes [29, 30].
The most common causes of LPI are lesions of the basal artery branches, long and short bypass arteries (43 %), of small paramedian arteries (34 %), and less often — occlusion of the basal artery (21 %) [31]. LPI is caused by lesions of the corticospinal, corticonuclear, corticopontine, and pontocerebellar tracts [16, 32]. Patients with LPI have a history of diabetes mellitus more often (50 %) compared to those with anterior circulation strokes [33]. Diabetes and arterial hypertension cause damage to the vessels of the microcirculatory channel (arteries and arterioles), the development of microangiopathy, and therefore LPI or microcirculatory pontine infarctions [34].
The pyramidal pathway at different levels of the pons has its own characteristics, and therefore the severity of the paresis depends on the level of pontine damage: movement disorders are more significant if an ischemic lesion affects the caudal part of the pons, less significant — the paramedian ventral area, and the rostral part of the pons, where the fibers of the pyramidal pathway pass diffusely.
Clinical presentation of LPI includes pure motor, pure sensory, and dysarthria-clumsy hand pontine syndromes [35]. Pure motor pontine stroke with hemiparesis or hemiplegia accounts for 10.2 % of all primary ischemic strokes and prevails among other pontine lacunar infarctions [36]. Pure sensory pontine strokes occur in case of ischemic lesions in the dorsal part of the pons [37]. Because the medial loop and the spinothalamic pathways are compatible here, the lesions lead to a disturbance of superficial and proprioceptive sensitivity. Often such patients complain of dysesthesia. Dysarthria-clumsy hand pontine syndrome occurs in case of lacunar infarction development in the basal parts of the pons and is accompanied by dysarthria and severe dysmetria of the arm and leg. It is believed that dysarthria is more common in patients with lesions of the left half of the pons [38]. In all LPI cases, the neurological deficit might be transient, lasting from seconds to 24 hours, causing transient ischemic attack [33, 39–41]. Effective secondary stroke prevention and early rehabilitation programs should be applied to all LPI patients to improve their quality of life and prevent disability and mortality [42–50].
A comparison of the neurological and functional reco-very showed that on the 21st day a favorable outcome (0–2 points) was achieved in all LPI patients: in 4 patients, there was a complete recovery of neurological functions (0 points by the mRS), in the other 11, insignificant neurological microsymptomatics persisted, which did not affect daily household activities (1 point on the mRS), and the recovery of neurological functions occurred within the first 7–14 days. Knowledge of the features of LPI is important, helping to promptly diagnose, select and apply adequate therapy and increase long-term functional prognosis.
Conclusions
The clinical course of LPI is extremely important when making a design about a patient’s diagnosis, management, and prognosis. We provided a comprehensive narrative review of the clinical features of LPI. Specific clinical and ima-ging features of LPI were determined, analyzed, compared, and described. LPI must be promptly diagnosed and treated to avoid high morbidity and mortality associated with it.
Prospects for further research. Future studies are needed on a larger number of patients to determine early clinical and imaging features of LPI, to promptly increase diagnosing and treatment of such patients to avoid high morbidity and mortality. It is also important to promote awareness of stroke prevention programs among patients and medical personnel.
Received 29.08.2022
Revised 11.09.2022
Accepted 15.09.2022
Список литературы
1. Malla G., Jillella D.V. Pontine Infarction. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2021 Jan-. Avai-lable from: https://www.ncbi.nlm.nih.gov/books/NBK554418.
2. Prokopiv M.M., Yevtushenko S.K., Fartushna O.Ye. Classification of pontine infarctions. International Neurological Journal. 2022. 18(1). 30-34. doi: 10.22141/2224-0713.18.1.2022.926.
3. Prokopiv M.M., Slabkiy G.O., Fartushna O.Y. Prospective analysis of the epidemiology of cerebrovascular disease and stroke among the adult population of Kyiv City, Ukraine. Wiad. Lek. 2021. 74 (10, p. II). 2599-2604. doi: 10.36740/WLek202110213.
4. Sciacca S., Lynch J., Davagnanam I., Barker R. Midbrain, Pons, and Medulla: Anatomy and Syndromes. Radiographics. 2019 Jul-Aug. 39(4). 1110-1125. doi: 10.1148/rg.2019180126.
5. Gowda S.N., De Jesus O. Brainstem Infarction. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2021 Jan-.
6. Prokopiv M., Fartushna O.Ye. Modern classification of posterior circulation stroke: clinical decision making and diagnosis (review). Georgian Med. News. 2021. 320. 96-100.
7. Erro M.E., Gállego J., Herrera M., Bermejo B. Isolated pontine infarcts: etiopathogenic mechanisms. Eur. J. Neurol. 2005. 12(12). 984-8. doi: 10.1111/j.1468-1331.2005.01119.x.
8. Kataoka S., Hori A., Shirakawa T., Hirose G. Paramedian pontine infarction. Neurological/topographical correlation. Stroke. 1997. 28(4). 809-15. doi: 10.1161/01.str.28.4.809.
9. Silverman I.E., Liu G.T., Volpe N.J., Galetta S.L. The crossed paralyses. The original brain-stem syndromes of Millard-Gubler, Foville, Weber, and Raymond-Cestan. Arch. Neurol. 1995 Jun. 52(6). 635-8. doi: 10.1001/archneur.1995.00540300117021.
10. Yevtushenko S.K., Filimonov D.A., Simonyan V.A. et al. The Main and New Risk Factors that Contribute to the Development of Ischemic Strokes in Young Adults. International Neurological Journal. 2013. 6(60). 92-100 (in Russian).
11. Prokopiv M.M., Fartushna O.Y. Classification of posterior circulation stroke: a narrative review of terminology and history. International Neurological Journal. 2021. 5(19). 11-19. doi: 10.22141/2224-0713.17.5.2021.238517.
12. Vinychuk S.M., Fartushna O.Ye. Case analysis of crossed pontine-cerebellar diaschisis in acute stroke patients. International Neurological Journal. 2018. 8(102). 20-24. doi: 10.22141/2224-0713.8.102.2018.153537.
13. Powers W.J., Rabinstein A.A., Ackerson T. et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019. 50(12). e344-e418. doi: 10.1161/STR.0000000000000211.
14. Prokopiv M.M., Yevtushenko S.K., Fartushna O.Ye. Clinical manifestations of acute pontine infarction: a narrative review. International Neurological Journal. 2022. 5.
15. Prokopiv M.M., Fartushna O.Y. Сlinical and imaging features of lacunar and non-lacunar subtypes of ischemic posterior circulation stroke. Wiad. Lek. 2021. 74(12). 3214-3220. doi: 10.36740/WLek202112116.
16. Prokopiv M.M., Vinychuk S.M. Vertebrobasilar strokes. Kyiv: PH “Avitsena”, 2021. 240 p. (in Ukrainian).
17. Prokopiv M.M., Rohoza S.V., Fartushna O.Ye. Lateral me-dullary infarction: a prospective hospital-based cohort study of clinical and imaging features and a case report in a white adult. Wiad. Lek. 2022. 75 (4, pt. 2). 938-943. doi: 10.36740/WLek202204203.
18. Prokopiv M.M., Fartushna O.Ye. Clinical syndromes of the thalamic stroke in the classical vascular territories: a prospective hospital-based cohort study. Wiad. Lek. 2020. 73(3). 489-493. doi: 10.36740/WLek202003115.
19. Vinychuk S.M., Prokopiv M.M., Trepet L.M., Fartushna O.Ye. Thalamic stroke outcomes: a prospective hospital-based cohort study. International Neurological Journal. 2019. 8(110). 23-27. doi: 10.22141/2224-0713.8.110.2019.187888.
20. Vinychuk S.M., Prokopiv M.M., Trepet L.M., Fartushna O.Ye. Clinical vascular syndromes of thalamic strokes in anterior and paramedian vascular territories: a prospective hospital-based cohort study. International Neurological Journal. 2020. 2(16). 7-12. doi: 10.22141/2224-0713.16.2.2020.200957.
21. Vinychuk S.M., Prokopiv M.M., Trepet L.M., Fartushna O.Ye. Clinical syndromes of a thalamic stroke in the lower lateral vascular territory: a prospective hospital-based cohort study. International Neurological Journal. 2020. 3(16). 1-6. doi: 10.22141/2224-0713.16.3.2020.203443.
22. Vinychuk S.M., Prokopiv M.M., Trepet L.M., Fartushna O.Ye. Clinical syndromes of thalamic strokes in posterolateral vascular territory: a prospective hospital-based cohort study. International Neurological Journal. 2020. 4(16). 8-12. doi: 10.22141/2224-0713.16.4.2020.207344.
23. Vinychuk S.M., Prokopiv M.M., Trepet L.M., Fartushna O.Ye. Clinical syndromes of thalamic stroke in the central vascular territory: a prospective hospital-based cohort study. International Neurological Journal. 2020. 5(16). 23-27.doi: 10.22141/2224-0713.16.5.2020.209245.
24. Vinychuk S.M., Fartushna O.Ye. Case analysis of crossed pontine-cerebellar diaschisis in acute stroke patients. International Neurological Journal. 2018. 8(102). 20-24. doi: 10.22141/2224-0713.8.102.2018.153537.
25. Yang L., Qin W., Li Y., Yang S., Gu H., Hu W. Differentiation of Pontine Infarction by Size. Open Med. (Wars.). 2020. 15. 160-166. doi: 10.1515/med-2020-0025.
26. Fartushna O.Ye. TIA with new ischemic lesions: clinical features and stroke risk for patients with different TIA pathological subtypes. Eur. J. Neurol. 2011. 18(2). 108.
27. Vinychuk S.M., Fartushna O.Ye. Terminology and definitions of transient ischemic attacks. A historical journey. International Neurological Journal. 2017. 4(90). 17-20. doi: 10.22141/2224-0713.4.90.2017.107257 (in Ukrainian).
28. Kumral E., Bayülkem G., Evyapan D. Clinical spectrum of pontine infarction. Clinical-MRI correlations. J. Neurol. 2002. 249(12). 1659-70. doi: 10.1007/s00415-002-0879-x.
29. Fartushna O.Ye., Vinychuk S.M. Transient ischemic attacks. Kyiv: PH “Avitsena”, 2014. 216 p. (in Ukrainian).
30. Fartushna O.Y., Vinychuk S.M. Brain injury in patients with acute TIA: clinical features for patients with different TIA subtypes. International Neurological Journal. 2017. 3(89). 34-39. doi: 10.22141/2224-0713.3.89.2017.104238.
31. Vemmos K.N., Spengos K., Tsivgoulis G. et al. Aetiopathogenesis and long-term outcome of isolated pontine infarcts. J. Neurol. 2005. 252. 212-217.
32. Yevtushenko S.K., Filimonov D.A., Simonyan V.A. et al. The Main and New Risk Factors that Contribute to the Development of Ischemic Strokes in Young Adults. International Neurological Journal. 2013. 6(60). 92-100 (in Russian).
33. Tun N.N., Arunagirinathan G., Munshi S.K., Pappachan J.M. Diabetes mellitus and stroke: A clinical update. World J. Diabetes. 2017 Jun 15. 8(6). 235-248. doi: 10.4239/wjd.v8.i6.235.
34. Vinychuk S.M., Fartushna O.Ye. Pathogenesis of transient ischemic attacks: the problem of subtypes. International Neurological Journal. 2017. 6(92). 11-16. doi: 10.22141/2224-0713.6.92.2017.111581 (in Ukrainian).
35. Saposnik G., Noel de Tilly L., Caplan L.R. Pontine Warning Syndrome. Arch. Neurol. 2008. 65(10). 1375-1377. doi: 10.1001/archneur.65.10.1375.
36. Ling L., Zhu L., Zeng J. et al. Pontine infarction with pure motor hemiparesis or hemiplegia: a prospective study. BMC Neurol. 2009. 9. 25. doi: 10.1186/1471-2377-9-25.
37. Prokopiv M.M. Vertebrobasilar infarctions: principles of classification, clinical and neuroimaging analysis and terminological definitions of the diagnosis. UMJ Heart & Vessels. 2019. 2(66). 7-17. doi: 10.30978/HV2019-2-7 (in Ukrainian).
38. Fisher C.M. A lacunar syndrome: the dysarthria-clumsy hand syndrome. Neurology. 1967. 17. 614-617.
39. Fartushna O.Ye., Prokopiv M.M. Actuality of the problem of cerebrovascular diseases, transient ischemic attacks, and improvement of their diagnostics in the health care system in Ukraine. Problems in military health care. Collection of Science of the Ukrainian Military Medical Academy. Kyiv: UMMA, 2007. 19. 335-342 (in Ukrainian).
40. Fartushna O.Ye., Vinychuk S.M. Epidemiology of transient ische-mic attacks in the structure of acute cerebrovascular disorders in Ukraine and in other countries. International Neurological Journal. 2017. 5 (91). 105-111. doi: 10.22141/2224-0713.5.91.2017.110863 (in Ukrainian).
41. Fartushna O.Y. Pathogenetic subtypes of transient ischemic attacks: features of neurological clinic, hemodynamics, and treatment. Kyiv, 2012. 217 p. (in Ukrainian).
42. Prokopiv M.M., Fartushna O.Ye., Mischenko V. Early and late rehabilitation after stroke in review: definition, classification, methods, and effectiveness. Acta Balneol. 2021. 4(166). 303-308. doi: 10.36740/ABAL202104110.
43. Vinychuk S.M., Fartushna O.Ye. Early rehabilitation after acute ischemic cerebrovascular events. International Neurological Journal. 2016. 8(86). 34-39. doi: 10.22141/2224-0713.8.86.2016.90909 (in Ukrainian).
44. Prokopiv M.M. The quality of life of metropolitan residents who have suffered a cerebral stroke. Ukraine. Nation’s Health. 2020. 1(58). 43-46. doi: 10.24144/2077-6594.1.2020.196420 (in Ukrainian).
45. Vinychuk S.M., Fartushna O.Ye. Differential treatment of transient ischemic attack is an effective way to prevent recurrent acute cerebral events. International Neurological Journal. 2014. 6(68). 87-92 (in Ukrainian).
46. Prior P.L., Suskin N. Exercise for stroke prevention. Stroke Vasc. Neurol. 2018 Jun 26. 3(2). 59-68. doi: 10.1136/svn-2018-000155.
47. Vinychuk S.M., Fartushna O.Y. History of the Kyiv Neurological School, about great teachers and wise predecessors (History of the Kyiv Neurological School). Kyiv: PH “Advance-Print”, 2015. 55 p. (in Russian).
48. Vinychuk S.M., Fartushna O.Ye. Diaschisis: brief historical review. International Neurological Journal. 2018. 4(98). 6-10. doi: 10.22141/2224-0713.4.98.2018.139419.
49. Vinychuk S.M., Fartushna O.Ye. Cerebrospinal and commissural diaschisis in acute stroke patients: case study. International Neurological Journal. 2018. 5(99). 20-25. doi: 10.22141/2224-0713.5.99.2018.142959.
50. Oza R., Rundell K., Garcellano M. Recurrent Ischemic Stroke: Strategies for Prevention. Am. Fam. Physician. 2017. 96(7). 436-440.
/8.jpg)