Журнал «Здоровье ребенка» Том 18, №4, 2023
Вернуться к номеру
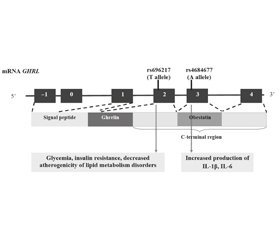
Асоціації варіантів гена GHRL із розвитком ожиріння та метаболічних порушень у дітей
Авторы: A. Abaturov, A. Nikulina
Dnipro State Medical University, Dnipro, Ukraine
Рубрики: Педиатрия/Неонатология
Разделы: Клинические исследования
Версия для печати
Актуальність. Однонуклеотидні варіанти (single nucleotide variant — SNV) гена греліну (GHRL) супроводжуються продукцією дефектного протеїну препрогреліну, що може призводити до розвитку ожиріння та метаболічних порушень. Мета: вивчити асоціації гена SNV GHRL із розвитком різних фенотипів ожиріння в дітей. Матеріали та методи. Обстежено 252 пацієнтів з ожирінням віком 6–18 років. Основну групу (n = 152) становили діти з метаболічно нездоровим ожирінням (МНО). Контрольну групу (n = 100) представили діти з метаболічно здоровим ожирінням (MЗO). У 31 дитини основної та 21 дитини контрольної групи проведено повногеномне секвенування (CeGat, Німеччина). Рівень інтерлейкіну (IL) 1β у сироватці крові визначали методом імунохемілюмінесцентного аналізу, IL-6 — методом імуноферментного аналізу (Synevo, Україна). Результати. Асоціація з розвитком MНO була вищою для T-алеля SNV rs696217 гена GHRL у здорових осіб (t = 2,31; p < 0.05) та пацієнтів з ожирінням (t = 2,06; p < 0,05). Генотип GT SNV rs696217 був пов’язаний з інсулінорезистентністю (r = 0,40; p < 0,05) у групі MНO і зворотно корелював з умістом холестерину (r = –0,45) та холестерину ліпопротеїнів низької щільності (r = –0,39). Генотип TA SNV rs4684677 корелював із рівнем IL-6 (r = 0,74) у групі MЗO та з IL-1β (r = 0,35) у групі MНO, p < 0,05. Профілактика трансформації MЗO в MНO визначається T-алелем SNV rs34911341 (t = 2,29, p < 0,05). Висновки. Міссенс-варіанти rs696217, rs4684677 гена GHRL є SNV, високо асоційованими з ожирінням та розвитком метаболічних порушень.
Background. Single nucleotide variants (SNVs) of the ghrelin (GHRL) gene are accompanied by the production of a defective preproghrelin protein, which can lead to the development of obesity and metabolic disorders. The purpose was to study the associations of SNVs of the GHRL gene in children with the development of various obesity phenotypes. Materials and methods. Two hundred and fifty-two obese children aged 6–18 years were examined. The main group (n = 152) was represented by patients with metabolically unhealthy obesity (MUO). The control group (n = 100) included children with metabolically healthy obesity (MHO). Whole genome sequencing (CeGat, Germany) was performed in 31 children of the main group and 21 controls. Serum levels of interleukin-1β were measured using a chemiluminescent immunoassay, interleukin-6 — by enzyme-linked immunosorbent assay (Synevo, Ukraine). Results. The association with the development of MUO was higher for the T allele of SNV rs696217 in healthy individuals (t = 2.31; p < 0.05) and obese patients (t = 2.06; p < 0.05). The GT genotype SNV rs696217 was associated with insulin resistance (r = 0.40; p < 0.05) in the MUO group and inversely correlated with levels of cholesterol (r = –0.45) and low-density lipoprotein cholesterol (r = –0.39) in children with MHO. The TA SNV rs4684677 genotype correlated with IL-6 levels (r = 0.74) in the MHO group and with IL-1β (r = 0.35) in children with MUO, p < 0.05. Prevention of the transformation of MHO into MUO is determined by the T allele SNV rs34911341 (t = 2.29, p < 0.05). Conclusions. The missense variants rs696217 and rs4684677 of the GHRL gene are SNVs highly associated with obesity and the development of metabolic disorders.
грелін; аналіз однонуклеотидних варіантів генів; діти; метаболічно нездорове ожиріння; метаболічно здорове ожиріння
ghrelin; analysis of single nucleotide gene variants; children; metabolically unhealthy obesity; metabolically healthy obesity
Для ознакомления с полным содержанием статьи необходимо оформить подписку на журнал.
- Abaturov A., Nikulina A. Genotype C/C 13910 of the Lactase Gene as a Risk Factor for the Formation of Insulin-Resistant Obesity in Children. Acta Medica (Hradec Kralove). 2019. 62(4). 150-155. doi: 10.14712/18059694.2020.4.
- Abaturov A., Nikulina A. Obesity in Children with Leptin Receptor Gene Polymorphisms. Acta Medica (Hradec Kralove). 2021. 64(3). 158-164. doi: 10.14712/18059694.2021.27.
- Alberti K.G., Zimmet P., Kaufman F. et al. The IDF consensus definition of the metabolic syndrome in children and adolescents. International Diabetes Federation. 2017. 17–19. Available from: https://www.idf.org/e-library/consensus-statements/61-idf-consensus-definition-of-metabolic-syndrome-in-children-and-adolescents.
- Alsulami S., Althagafi N., Hazazi E. et al. Obesity and Its Associations with Gender, Smoking, Consumption of Sugary Drinks, and Hour of Sleep Among King Abdulaziz University Students in Saudi Arabia. Diabetes Metab. Syndr. Obes. 2023 Apr 1. 16. 925-934. doi: 10.2147/DMSO.S405729.
- Becer E., Ergoren M.C. Dual Effect of the GHRL Gene Variant in the Molecular Pathogenesis of Obesity. Balkan J. Med. Genet. 2021 Jul 27. 24(1). 27-34. doi: 10.2478/bjmg-2021-0011.
- Bing C., Ambye L., Fenger M. et al. Large-scale studies of the Leu72Met polymorphism of the ghrelin gene in relation to the metabo–lic syndrome and associated quantitative traits. Diabet. Med. 2005 Sep. 22(9). 1157-60. doi: 10.1111/j.1464-5491.2005.01575.x.
- Buraczynska M., Golacki J., Zaluska W. Leu72Met Polymorphism in Ghrelin Gene: A Potential Risk Factor for Hypertension in Type 2 Diabetes Patients. Diabetes Metab. Syndr. Obes. 2023 Mar 1. 16. 557-564. doi: 10.2147/DMSO.S393373.
- Chagnon Y.C., Rankinen T., Snyder E.E. et al. The human obesity gene map: the 2002 update. Obes. Res. 2003 Mar. 11(3). 313-67. doi: 10.1038/oby.2003.47.
- Cornejo M.P., Mustafá E.R., Cassano D. et al. The ups and downs of growth hormone secretagogue receptor signaling. FEBS J. 2021 Dec. 288(24). 7213-7229. doi: 10.1111/febs.15718.
- Crovesy L., Rosado E.L. Interaction between genes involved in energy intake regulation and diet in obesity. Nutrition. 2019 Nov-Dec. 67–68. 110547. doi: 10.1016/j.nut.2019.06.027.
- Deelen P., Bonder M.J., van der Velde K.J. et al. Genotype harmonizer: automatic strand alignment and format conversion for genotype data integration. BMC Res. Notes. 2014. 7. 901. doi: 10.1186/1756-0500-7-901.
- Deignan J.L., Chung W.K., Kearney H.M. et al. Points to consi–der in the reevaluation and reanalysis of genomic test results: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2019. 21(6). 1267-1270. doi: 10.1038/s41436-019-0478-1.
- Draznin B., Aroda V.R., Bakris G. et al. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: Standards of Medical Care in Diabetes — 2022. Diabetes Care. 2022. 45 (Suppl. 1). 83-96. doi: 10.2337/dc22-S006.
- Elkins C., Fruh Sh., Jones L. et al. Clinical Practice Recommendations for Pediatric Dyslipidemia. Journal of Pediatric Health Care. 2019. 33(4). 494-504. doi: 10.1016/j.pedhc.2019.02.009.
- Flynn J.T., Kaelber D.C., Baker-Smith C.M. et al.; Subcommittee on screening and management of high blood pressure in children. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017 Sep. 140(3). e20171904. doi: 10.1542/peds.2017-1904.
- Goodarzi M.O. Genetics of obesity: what genetic association stu–dies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018 Mar. 6(3). 223-236. doi: 10.1016/S2213-8587(17)30200-0.
- Gross J.D., Zhou Y., Barak L.S., Caron M.G. Ghrelin receptor signaling in health and disease: a biased view. Trends Endocrinol. Metab. 2023 Feb. 34(2). 106-118. doi: 10.1016/j.tem.2022.12.001.
- Gueorguiev M., Lecoeur C., Meyre D. et al. Association studies on ghrelin and ghrelin receptor gene polymorphisms with obesity. Obesity (Silver Spring). 2009 Apr. 17(4). 745-54. doi: 10.1038/oby.2008.589.
- Hassouna R., Zizzari P., Viltart O. et al. A natural variant of obestatin, Q90L, inhibits ghrelin’s action on food intake and GH secretion and targets NPY and GHRH neurons in mice. PLoS One. 2012. 7(12). e51135. doi: 10.1371/journal.pone.0051135.
- Hongshan J., Rong L., Shou-Wei D. et al. Skewer: a fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinformatics. 2014. 15. 182. doi: 10.1186/1471-2105-15-182.
- gnomAD browser. Available from: https://gnomad.broadinstitute.org/variant/3-10334546-C-T?dataset=gnomad_r2_1.
- Jebeile H., Kelly A.S., O’Malley G. et al. Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022 May. 10(5). 351-365. doi: 10.1016/S2213-8587(22)00047-X.
- Joatar F.E., Al Qarni A.A., Ali M.E. et al. Leu72Met and Other Intronic Polymorphisms in the GHRL and GHSR Genes Are Not Associated with Type 2 Diabetes Mellitus, Insulin Resistance, or Serum Ghrelin Levels in a Saudi Population. Endocrinol. Metab. (Seoul). 2017 Sep. 32(3). 360-369. doi: 10.3803/EnM.2017.32.3.360.
- Kojima M., Hosoda H., Date Y. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999 Dec 9. 402(6762). 656-60. doi: 10.1038/45230.
- Kojima M., Kangawa K. Ghrelin: structure and function. Physiol. Rev. 2005 Apr. 85(2). 495-522. doi: 10.1152/physrev.00012.2004.
- Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009. 25(14). 1754-1760. doi: 10.1093/bioinformatics/btp324.
- Liu J., Liu J., Tian L.M. et al. Association of ghrelin Leu72Met polymorphism with type 2 diabetes mellitus in Chinese population. Gene. 2012 Aug 10. 504(2). 309-12. doi: 10.1016/j.gene.2012.03.025.
- Lu X., Huang L., Huang Z. et al. LEAP-2: An Emerging Endogenous Ghrelin Receptor Antagonist in the Pathophysiology of Obesity. Front. Endocrinol. (Lausanne). 2021 Aug 24. 12. 717544. doi: 10.3389/fendo.2021.717544.
- Lv Y., Liang T., Wang G., Li Z. Ghrelin, a gastrointestinal hormone, regulates energy balance and lipid metabolism. Biosci. Rep. 2018 Sep 25. 38(5). BSR20181061. doi: 10.1042/BSR20181061.
- Miraglia del Giudice E., Santoro N., Cirillo G. et al. Molecular screening of the ghrelin gene in Italian obese children: the Leu72Met variant is associated with an earlier onset of obesity. Int. J. Obes. Relat. Metab. Disord. 2004 Mar. 28(3). 447-50. doi: 10.1038/sj.ijo.0802572.
- Mose L.E., Wilkerson M.D., Hayes D.N. et al. ABRA: improved coding indel detection via assembly-based realignment. Bioinformatics. 2014. 30(19). 2813-2815. doi: 10.1093/bioinformatics/btu376.
- Panera N., Mandato C., Crudele A. et al. Genetics, epigenetics and transgenerational transmission of obesity in children. Front. Endocrinol. (Lausanne). 2022 Nov 14. 13. 1006008. doi: 10.3389/fendo.2022.1006008.
- Peplies J., Börnhorst C., Günther K. et al.; IDEFICS consortium. Longitudinal associations of lifestyle factors and weight status with insulin resistance (HOMA-IR) in preadolescent children: the large prospective cohort study IDEFICS. Int. J. Behav. Nutr. Phys. Act. 2016 Sep 2. 13(1). 97. doi: 10.1186/s12966-016-0424-4.
- Pérusse L., Rankinen T., Zuberi A. et al. The human obesity gene map: the 2004 update. Obes. Res. 2005 Mar. 13(3). 381-490. doi: 10.1038/oby.2005.50.
- Poher A.L., Tschöp M.H., Müller T.D. Ghrelin regulation of glucose metabolism. Peptides. 2018 Feb. 100. 236-242. doi: 10.1016/j.peptides.2017.12.015.
- Pradhan G., Samson S.L., Sun Y. Ghrelin: much more than a hunger hormone. Curr. Opin. Clin. Nutr. Metab. Care. 2013 Nov. 16(6). 619-24. doi: 10.1097/MCO.0b013e328365b9be.
- Rankinen T., Pérusse L., Weisnagel S.J. et al. The human obesity gene map: the 2001 update. Obes. Res. 2002 Mar. 10(3). 196-243. doi: 10.1038/oby.2002.30.
- Rankinen T., Zuberi A., Chagnon Y.C. et al. The human obesity gene map: the 2005 update. Obesity (Silver Spring). 2006 Apr. 14(4). 529-644. doi: 10.1038/oby.2006.71.
- RefSeq: NCBI Reference Sequence Database. Available from: https://www.ncbi.nlm.nih.gov/refseq.
- Richards S., Aziz N., Bale S. et al.; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015 May. 17(5). 405-24. doi: 10.1038/gim.2015.30.
- Rivera-León E.A., Llamas-Covarrubias M.A., Sánchez-Enríquez S. et al. Leu72Met polymorphism of GHRL gene decreases susceptibility to type 2 diabetes mellitus in a Mexican population. BMC Endocr. Disord. 2020 Jul 22. 20(1). 109. doi: 10.1186/s12902-020-00596-3.
- Saliba L.F., Reis R.S., Brownson R.C. et al. Obesity-related gene ADRB2, ADRB3 and GHRL polymorphisms and the response to a weight loss diet intervention in adult women. Genet. Mol. Biol. 2014 Mar. 37(1). 15-22. doi: 10.1590/s1415-47572014000100005.
- Seim I., Collet C., Herington A.C. et al. Revised genomic structure of the human ghrelin gene and identification of novel exons, alternative splice variants and natural antisense transcripts. BMC Genomics. 2007 Aug 30. 8. 298. doi: 10.1186/1471-2164-8-298.
- Skrivankova V.W., Richmond R.C., Woolf B.A.R. et al. Streng–thening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA. 2021. 326(16). 1614-1621. doi: 10.1001/jama.2021.18236.
- Smith A., Woodside B., Abizaid A. Ghrelin and the Control of Energy Balance in Females. Front. Endocrinol. (Lausanne). 2022 Jul 15. 13. 904754. doi: 10.3389/fendo.2022.904754.
- Snyder E.E., Walts B., Pérusse L. et al. The human obesity gene map: the 2003 update. Obes. Res. 2004 Mar. 12(3). 369-439. doi: 10.1038/oby.2004.47.
- Su M., Qiu L., Wang Q. et al. Associations of Leu72Met Polymorphism of Preproghrelin with Ratios of Plasma Lipids Are Diversified by a High-Carbohydrate Diet in Healthy Chinese Adolescents. Ann. Nutr. Metab. 2015. 67(4). 236-42. doi: 10.1159/000440777.
- Tabaeian S.P., Mahmoudi T., Sabzikarian M. et al. The Leu72Met (rs696217 G>T) Polymorphism of the Ghrelin Gene Might Be a Protective Factor for Nonalcoholic Fatty Liver Disease. J. Gastrointestin. Liver Dis. 2021 Jun 19. 30(2). 233-239. doi: 10.15403/jgld-2703.
- Ukkola O., Ravussin E., Jacobson P. et al. Role of ghrelin polymorphisms in obesity based on three different studies. Obes. Res. 2002 Aug. 10(8). 782-91. doi: 10.1038/oby.2002.106.
- Villarreal D., Pradhan G., Zhou Y. et al. Diverse and Complementary Effects of Ghrelin and Obestatin. Biomolecules. 2022 Mar 29. 12(4). 517. doi: 10.3390/biom12040517.
- Xing Y.X., Yang L., Kuang H.Y. et al. Function of obestatin in the digestive system. Nutrition. 2017 Feb. 34. 21-28. doi: 10.1016/j.nut.2016.08.009.
- Yadegari M., Zare-Feyzabadi R., Zakariaeiseraji M. et al. Interaction between the genetic variant of rs696217-ghrelin and food intake and obesity and dyslipidemia. Ann. Hum. Genet. 2022 Jan. 86(1). 14-23. doi: 10.1111/ahg.12443.
- Yanagi S., Sato T., Kangawa K. et al. The Homeostatic Force of Ghrelin. Cell. Metab. 2018 Apr 3. 27(4). 786-804. doi: 10.1016/j.cmet.2018.02.008.
- Zhang S., Zhai G., Zhang J. et al. Ghrelin and obestatin plasma levels and ghrelin/obestatin prepropeptide gene polymorphisms in small for gestational age infants. J. Int. Med. Res. 2014 Dec. 42(6). 1232-42. doi: 10.1177/0300060514533525.