Международный эндокринологический журнал Том 20, №3, 2024
Вернуться к номеру
Прогнозування ефективності реабілітації в пацієнтів із цукровим діабетом 2-го типу та діабетичною полінейропатією
Авторы: T.H. Bakaliuk (1), N.R. Makarchuk (1), H.O. Stelmakh (1), V.I. Pankiv (2), I.I. Kamyshna (1)
(1) - I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
(2) - Ukrainian Scientific and Practical Centre of Endocrine Surgery, Transplantation of Endocrine Organs and Tissues of the Ministry of Health of Ukraine, Kyiv, Ukraine
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
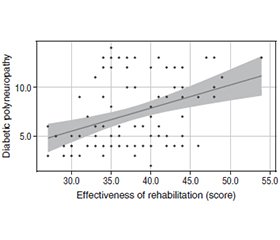
Актуальність. Прогнозування ефективності реабілітації в пацієнтів із діабетичною полінейропатією (ДПН) при цукровому діабеті 2-го типу (ЦД2) має велике значення в сучасній клінічній практиці. Зважаючи на поширеність ЦД2 і його ускладнень, у тому числі ДПН, розробка прогностичних моделей дозволить персоналізувати лікувальні підходи, оптимізувати реабілітаційні програми та підвищити якість життя пацієнтів. Інтеграція новітніх методів аналізу даних та молекулярно-біологічних підходів у прогностичні моделі сприятиме розвитку інноваційних стратегій реабілітації та покращенню результатів лікування в цієї важливої категорії пацієнтів. Мета роботи: запропонувати багатофакторну регресійну математичну модель прогнозування ефективності реабілітації діабетичної полінейропатії. Матеріали та методи. Для побудови прогностичної моделі за допомогою багатофакторного регресійного аналізу обстежено 95 хворих на ЦД2. Для перевірки якості моделі використовували критерій Нейджелкерка (R2). Результати. Аналіз виявив значущі зв’язки між різними факторами та ефективністю реабілітації пацієнтів із діабетичною полінейропатією. Зокрема, збільшення віку пов’язане з передбачуваним зниженням ефективності реабілітації на 0,103. Крім того, збільшення тривалості цукрового діабету пов’язане з очікуваним зниженням ефективності реабілітації від 1,341 до 3,732 залежно від діапазону тривалості. Так само працевлаштування, тютюнокуріння, індекс маси тіла, рівень глікованого гемоглобіну (HbA1c), рухливість, здатність до самообслуговування, повсякденна діяльність, біль/дискомфорт, тривога/депресія, сенсорна чутливість, показники DN4 та профіль ліпідів були значущо пов’язані зі змінами в ефективності реабілітації. Регресійна модель продемонструвала високу ефективність та прийнятність із кореляційним коефіцієнтом (rxy) 0,997, що свідчить про сильний функціональний зв’язок. Крім того, модель була статистично значущою (p < 0,001). Ці результати підкреслюють важливість врахування багатьох факторів при прогнозуванні результатів реабілітації в пацієнтів із ДПН і підкреслюють потенційну корисність розробленої моделі в клінічній практиці. Висновки. Математична модель прогнозування ефективності реабілітації ДПН у хворих на ЦД2 демонструє високу прийнятність, якість та ефективність. Використання цієї моделі, що враховує 99,5 % факторів, дозволить підвищити точність та своєчасність реабілітації пацієнтів, покращити результати лікування, проводити регулярний моніторинг хворих із високим ризиком ускладнень, сприяти розробці інформаційних листів та адаптованих програм профілактики ДПН у хворих на ЦД2, а також створенню відповідних медичних калькуляторів та інформаційних систем.
Background. Predicting the effectiveness of rehabilitation in patients with diabetic polyneuropathy (DPN) and type 2 diabetes mellitus is of great importance in modern clinical practice. Given the prevalence of diabetes and its complications, including DPN, the development of predictive models will allow for personalized treatment approaches, optimization of rehabilitation programs, and improvement in the quality of life for patients. Integrating state-of-the-art data analysis methods and molecular-biological approaches into predictive models will contribute to the development of innovative rehabilitation strategies and improve treatment outcomes in this important patient population. The purpose of the study was to propose a multifactorial regression mathematical model for predicting the effectiveness of diabetic polyneuropathy rehabilitation. Materials and methods. Ninety-five patients with type 2 diabetes and DPN were examined to construct a predictive model of rehabilitation effectiveness using multiple regression analysis. The quality of the model was evaluated using the Nagelkerke criterion (R2). Results. The analysis revealed several significant associations between various factors and the effectiveness of rehabilitation in DPN patients. Specifically, an increase in age was associated with a predicted decrease in rehabilitation effectiveness by 0.103. Moreover, each increase in the duration of diabetes mellitus was associated with an expected decrease in rehabilitation effectiveness, ranging from 1.341 to 3.732 depending on the duration range. Similarly, changes in tobacco smoking, employment status, body mass index, glycated hemoglobin levels, mobility, self-care, usual activities, pain/discomfort, anxiety/depression, sensory sensitivities, DN4 scores, and lipid profile were all significantly associated with variations in rehabilitation effectiveness. The regression model demonstrated high explanatory power, with an observed correlation coefficient (rxy) of 0.997, indicating a strong functional relationship. Furthermore, the model was statistically significant (p < 0.001), suggesting that the identified predictors collectively explain 99.5 % of the observed variance in rehabilitation effectiveness. These findings underscore the importance of considering multiple factors when predicting rehabilitation outcomes in DPN patients and highlight the potential utility of the developed model in clinical practice. Conclusions. The proposed mathematical model for predicting the effectiveness of rehabilitation in type 2 diabetes patients with DPN demonstrates high acceptability, quality, and effectiveness. The application of this model, considering 99.5 % of DPN factors, will enhance the accuracy and timeliness of rehabilitation, improve treatment outcomes, facilitate regular monitoring of patients at high risk of complications, promote the development of informational leaflets and adapted programs for DPN prevention in type 2 diabetes patients, and facilitate the creation of relevant medical calculators and informational systems.
цукровий діабет; діабетична полінейропатія; реабілітація; фізична терапія; прогнозування
diabetes mellitus; diabetic polyneuropathy; rehabilitation; physical therapy; prognosis
Introduction
Materials and methods
Results
/9.jpg)
Discussion
Conclusions
- Zhu J, Hu Z, Luo Y, Liu Y, Luo W, et al. Diabetic periphe–ral neuropathy: pathogenetic mechanisms and treatment. Front Endocrinol (Lausanne). 2024 Jan 9;14:1265372. doi: 10.3389/fendo.2023.1265372.
- Zuidema X, de Galan B, Brouwer B, Cohen SP, Eldabe S, et al. 4. Painful diabetic polyneuropathy. Pain Pract. 2024 Feb;24(2):308-320. doi: 10.1111/papr.13308.
- Amara F, Hafez S, Orabi A, El Etriby A, Abdel Rahim AA, et al. Review of Diabetic Polyneuropathy: Pathogenesis, Diagnosis and Management According to the Consensus of Egyptian Experts. Curr Diabetes Rev. 2019;15(4):340-345. doi: 10.2174/1573399815666190226150402.
- Bakaliuk TG, Маkarchuk NR, Stelmakh HO, Martynyuk LP, Strashko YY, Levytska LV. Quality of life in patients with diabe–tic polyneuropathy with increased physical activity. Wiad Lek. 2021;74(6):1302-1306. doi: 10.36740/WLek202106102.
- Xu Q, Lin Y, He Y, Zhou X, Liu J, et al. Predictive models for perceived convenience of accessing outdoor activities among elderly with physical disabilities in rural China. BMC Public Health. 2024 Mar 12;24(1):776. doi: 10.1186/s12889-024-18311-5.
- Vieira JEA, Ferreira AS, Monnerat LB, Cal MSD, Ghetti ATA, et al. Prediction models for physical function in COVID-19 survivors. J Bodyw Mov Ther. 2024 Jan;37:70-75. doi: 10.1016/j.jbmt.2023.11.002.
- Kamyshna II, Pavlovych LB, Kamyshnyi AM. Prediction of the cognitive impairment development in patients with autoimmune thyroiditis and hypothyroidism. Endocr Regul. 2022 Jul 13;56(3):178-189. doi: 10.2478/enr-2022-0019.
- Yang Z, Zhang Y, Chen R, Huang Y, Ji L, et al. Simple tests to screen for diabetic peripheral neuropathy. Cochrane Database Syst Rev. 2018 Jul 30;2018(7):CD010975. doi: 10.1002/14651858.CD010975.pub2.
- Oggiam DS, Jorgetto JV, Chinini GL, Kusahara DM, Gamba MA. Distal Symmetric Polyneuropathy Pain in Diabetes Mellitus. Aquichan. 2021;21(3):e213X. doi: 10.5294/aqui.2021.21.3.7.
- Jong Chul Won, Tae Sun Park. Recent Advances in Diagnostic Strategies for Diabetic Peripheral Neuropathy. Endocrinology and Metabolism. 2016;31(2):230-238.
- Sykioti P, Zis P, Vadalouca A, et al. Estimating the diagnostic value of DN4 versions. Pain Pract. 2014;14(1):95.
- Dakin H, Abel L, Burns R, et al. Review and critical appraisal of studies mapping from quality of life or clinical measures to EQ-5D: an online database and application of the MAPS statement. Health Qual Life Outcomes. 2018;16(1):31.
- Khdour MR. Treatment of diabetic peripheral neuropathy: a review. J Pharm Pharmacol. 2020 Jul;72(7):863-872. doi: 10.1111/jphp.13241.
- Ardeleanu V, Toma A, Pafili K, Papanas N, Motofei I, et al. Current Pharmacological Treatment of Painful Diabetic Neuropathy: A Narrative Review. Medicina (Kaunas). 2020 Jan 9;56(1):25. doi: 10.3390/medicina56010025.
- Rafiullah M, Siddiqui K. Pharmacological Treatment of Diabetic Peripheral Neuropathy: An Update. CNS Neurol Disord Drug Targets. 2022;21(10):884-900. doi: 10.2174/1871527320666210303111939.
- Jang HN, Oh TJ. Pharmacological and Nonpharmacological Treatments for Painful Diabetic Peripheral Neuropathy. Diabetes Metab J. 2023 Nov;47(6):743-756. doi: 10.4093/dmj.2023.0018.
- Akbari NJ, Naimi SS. The effect of exercise therapy on ba–lance in patients with diabetic peripheral neuropathy: a systematic review. J Diabetes Metab Disord. 2022 Jul 4;21(2):1861-1871. doi: 10.1007/s40200-022-01077-1.
- Dixit S, Gular K, Asiri F. Effect of diverse physical rehabili–tative interventions on static postural control in diabetic periphe–ral neuropathy: a systematic review. Physiother Theory Pract. 2020 Jun;36(6):679-690. doi: 10.1080/09593985.2018.1491078.
- Yorek M. Treatment for Diabetic Peripheral Neuropathy: What have we Learned from Animal Models? Curr Diabetes Rev. 2022;18(5):e040521193121. doi: 10.2174/1573399817666210504101609.
- Lee KA, Park TS, Jin HY. Non-glucose risk factors in the pathogenesis of diabetic peripheral neuropathy. Endocrine. 2020 Dec;70(3):465-478. doi: 10.1007/s12020-020-02473-4.
- Pankiv V. Treatment of neurological complications in patients with type 2 diabetes mellitus at the stage of rehabilitation after COVID-19. International Journal of Endocrinology (Ukraine). 2021;17(6):491-495. doi: 10.22141/2224-0721.17.6.2021.243214.

/8.jpg)
/8_2.jpg)
/9_2.jpg)
/10.jpg)