Журнал «Боль. Суставы. Позвоночник» Том 15, №1, 2025
Вернуться к номеру
Визначення індексу ризику переломів Колліса на основі рентгенівських знімків за допомогою програми комп’ютерної оцінки
Авторы: Wisam A. Hussein, Hussain J. AlKhatteib, Jawad K. Abbas
University of Kufa, Najaf, Iraq
Рубрики: Ревматология, Травматология и ортопедия
Разделы: Клинические исследования
Версия для печати
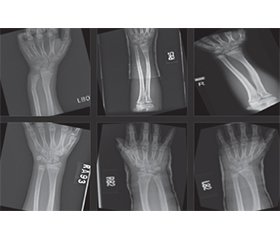
Актуальність. Моделювання прогностичного індексу ризику переломів Колліса за допомогою аналізу рентгенівських зображень є вирішальним при застосуванні в ортопедії, оскільки переломи цієї локалізації мають суттєвий тягар для здоров’я та економіки, особливо в осіб літнього віку. Попередні дослідження встановили зв’язок між зниженням мінеральної щільності кісткової тканини та підвищеним ризиком переломів цієї локалізації. Мета дослідження: оцінка комп’ютерної моделі для прогнозування ризику виникнення переломів Колліса при будь-якому падінні або фізичному навантаженні на зап’ясток. Матеріали та методи. Ця модель отримана шляхом аналізу кількісних характеристик рентгенівських зображень передпліччя. Прогностична модель була створена розробником проєкту AIT з використанням Python 3.12, PHP і JS, html. Модель AIT233 була застована згідно з некомерційною ліцензією на наукові дослідження. Рентгенівські знімки передпліччя здорових осіб (без переломів, контрольна група) і осіб з переломами Колліса були завантажені з ліцензованого репозиторію. Із застосуванням методів порогового визначення зображення, нормалізації та реєстрації було отримано та проаналізовано знімки кісток за допомогою алгоритмів комп’ютерної оцінки. Результати. Порівняльний аналіз інтенсивності зображення передпліччя в осіб контрольної групи та пацієнтів з переломами Колліса дав відносний індекс інтенсивності кісток 1,09 (82 ± 5 проти 75 ± 6 пікселів відповідно). Результати виявили статистично значущі відмінності між групами (p < 0,05), що свідчить про те, що інтенсивність зображення є потенційним предиктором ризику переломів. Розрахований показник ризику на основі інтенсивності зображення продемонстрував позитивний зв’язок із виникненням переломів Колліса. Висновок. Отримані нами результати є основою для розробки надійного інструменту прогнозування, який може допомогти в профілактиці та лікуванні хворих з переломом Колліса.
Background. Modelling a predictive risk index for Colles fractures using X-ray image analysis is a crucial application in orthopaedics since these fractures have essential health and economic burdens, particularly among the elderly. Previous research has established a correlation between decreased bone mineral density and the elevated risk of these fractures. This study purposed to assess a computer vision model for predicting the risk of Colles fractures occurrence upon any fall or physical stress on the wrist. Materials and methods. This model was obtained by analyzing quantitative characteristics extracted from forearm X-ray images. A predictive model was designed by the AIT project developer using Python 3.12, PHP and JS, html. The use of the AIT233 model was granted for this study under a non-profit scientific research license. Forearm X-ray datasets for the normal (without fracture, control group) and Colles fracture individuals were downloaded from a licensed repository. By implementing image thresholding, normalization and registration techniques, validated bone images were obtained and analyzed using computer vision algorithms. Results. A comparative analysis of forearm image intensity between subjects from the control group and patients with Colles fractures resulted in a 1.09 relative index of bone intensity (mean 82 ± 5 vs. 75 ± 6 pixels, respectively). The results showed statistically significant differences (p < 0.05), suggesting that image intensity is a potential predictor of fracture risk. A calculated risk score based on image intensity demonstrated a positive association with the occurrence of Colles fracture. Conclusions. The results provide the basis for developing a robust predictive tool that can aid in the prevention and management of Colles fractures.
перелом Колліса; індекс ризику; методи порогової обробки; рентгендослідження
Colles fracture; risk index; thresholding; Х-ray
Introduction
Models and datasets
/14.jpg)
Results
/17.jpg)
Discussion
Conclusions
- Obermeyer Z, Emanuel EJ. Predicting the Future — Big Data, Machine Learning, and Clinical Medicine. N Engl J Med. 2016 Sep 29;375(13):1216-1219. doi: 10.1056/NEJMp1606181.
- Brogren E, Petranek M, Atroshi I. Incidence and characteristics of distal radius fractures in a southern Swe–dish region. BMC Musculoskelet Disord. 2007 May 31;8:48. doi: 10.1186/1471-2474-8-48.
- Çiçek Ö, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger O. 3D U-Net: Learning dense volumetric segmentation from sparse annotation. In: Ourselin S, Joskowicz L, Sabuncu M, Unal G, Wells W, editors. Medical Image Computing and Computer-Assisted Intervention — –MICCAI 2016. Series: Lecture Notes in Computer Science, vol. 9901. Cham: Springer; 2016. 424-432 pp. doi: 10.1007/978-3-319-46723-8_49.
- Engelke K, Libanati C, Fuerst T, Zysset P, Genant HK. Advanced CT based in vivo methods for the assessment of bone density, structure, and strength. Curr Osteoporos Rep. 2013 Sep;11(3):246-255. doi: 10.1007/s11914-013-0147-2.
- Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017 Dec;42:60-88. doi: 10.1016/j.media.2017.07.005.
- Kanis JA, Harvey NC, Johansson H, Odén A, Leslie WD, McCloskey EV. FRAX and fracture prediction without bone mineral density. Climacteric. 2015;18(Suppl 2):2-9. doi: 10.3109/13697137.2015.1092342.
- Kanis JA, Johansson H, Oden A, McCloskey EV. Assessment of fracture risk. Eur J Radiol. 2009 Sep;71(3):392-397. doi: 10.1016/j.ejrad.2008.04.061.
- Kapur JN, Sahoo PK, Wong AKC. A new –method for gray-level picture thresholding using the entropy of the histogram. Comput Vis Graph Image Process. 1985 Mar;29(3):273-285. doi: 10.1016/0734-189X(85)90125-2.
- Hassanabadi N, Berger C, Papaioannou A, et al. Variation in bone mineral density and fractures over 20 years among Canadians: a comparison of the Canadian Multicenter Osteoporosis Study and the Canadian Longitudinal Study on Aging. Osteoporos Int. 2023 Feb;34(2):357-367. doi: 10.1007/s00198-022-06623-4.
- Sahoo PK, Soltani S, Wong AKC, Chen YC. A survey of thresholding techniques. Comput Vision Graph Image Process. 1988;41(2):233-260. doi: 10.1016/0734-189X(88)90022-9.
- Liu H, Durongbhan P, Davey CE, Stok KS. –Ima–ge Registration in Longitudinal Bone Assessment –Using Computed Tomography. Curr Osteoporos Rep. 2023 Aug;21(4):372-385. doi: 10.1007/s11914-023-00795-6.
- Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. In: Navab N, Hornegger J, Wells W, Frangi A, editors. Medical Image Computing and Computer-Assisted Intervention — MICCAI 2015. Series: Lecture Notes in Computer Science, vol. 9351. Cham: Springer; 2015. 234-241 pp. doi: 10.1007/978-3-319-24574-4_28.
- Kanis JA, Harvey NC, McCloskey E, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int. 2020 Jan;31(1):1-12. doi: 10.1007/s00198-019-05176-3.
- Sotiras A, Davatzikos C, Paragios N. Defor–mable medical image registration: A survey. IEEE Trans Med Imaging. 2013;32(7):1153-1190. doi: 10.1109/TMI.2013.2265603.
- Darzi F, Bocklitz T. A Review of Medical –Image Registration for Different Modalities. Bioengineering (Basel). 2024 Aug 2;11(8):786. doi: 10.3390/bioengine–ering11080786.
- Sauvola J, Pietikäinen M. Adaptive document image binarization. Pattern Recognit. 2000;33(2):225-236. doi: 10.1016/S0031-3203(99)00055-2.
- Gonzalez RC, Woods RE. Digital Image Processing. 4th ed. New York: Pearson; 2018.
- Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a dataset via the gap statistic. Journal of the Royal Statistical Society: Series B (Statistical Methodology). 2001;63(2):411-423. doi: 10.1111/1467-9868.00293.
- Russ JC, Neal FB. The Image Processing Handbook. 7th ed. Boca Raton, FL: CRC Press; 2016. 1053 p. doi: 10.1201/b18983.
- Sonka M, Hlavac V, Boyle R. Image Processing, Analysis, and Machine Vision. 4th ed. Boston, MA: Cengage Learning; 2014. 920 p.
- Haralick RM, Sternberg SR, Zhuang X. Image ana–lysis using mathematical morphology. IEEE Trans Pattern Anal Mach Intell. 1987 Apr;9(4):532-550. doi: 10.1109/tpami.1987.4767941.
- Zitová B, Flusser J. Image registration methods: A survey. Image Vis Comput. 2003;21(11):977-1000. doi: 10.1016/S0262-8856(03)00137-9.
- Xu H, Xue T, Fan J, et al. Medical Image Registration Meets Vision Foundation Model: Prototype Learning and Contour Awareness. arXiv. 2025 Feb:2502.11440v1[cs CV]. doi: 10.48550/arXiv.2502.11440.
- Kanis JA, Cooper C, Rizzoli R, Reginster JY; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019 Jan;30(1):3-44. doi: 10.1007/s00198-018-4704-5.
- Cosman F, de Beur SJ, LeBoff MS, et al.; National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014 Oct;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Baines AJ, Babazadeh-Naseri A, Dunbar NJ, Le–wis VO, Fregly BJ. Bilateral asymmetry of bone density adjacent to pelvic sarcomas: A retrospective study using computed tomography. J Orthop Res. 2022 Mar;40(3):644-653. doi: 10.1002/jor.25067.
- Tsai PH, Wong CC, Chan WP. Radial T2 mapping reveals early meniscal abnormalities in patients with knee osteoarthritis. Eur Radiol. 2022 Aug;32(8):5642-5649. doi: 10.1007/s00330-022-08641-6.
- Karimi D, Zeng Q, Mathur P, et al. Accurate and robust deep learning-based segmentation of the prostate clinical target volume in ultrasound images. Med Image Anal. 2019 Oct;57:186-196. doi: 10.1016/j.media.2019.07.005.
- Matharu GS, Judge A, Murray DW, Pandit HG. Prevalence of and Risk Factors for Hip Resurfacing Revision: A Cohort Study іnto the Second Decade After the Operation. J Bone Joint Surg Am. 2016 Sep 7;98(17):1444-1452. doi: 10.2106/JBJS.15.01234.
- Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002 May 18;359(9319):1761-1767. doi: 10.1016/S0140-6736(02)08657-9.
- Sözen T, Özışık L, Başaran NÇ. An overview and management of osteoporosis. Eur J Rheumatol. 2017 Mar;4(1):46-56. doi: 10.5152/eurjrheum.2016.048.
- Colles Fracture Predictor. Available from: https://ait-sciences.com/wp-content/uploads/2025/02/FracturePredictor17-4.html.

/16.jpg)
/16_2.jpg)