Журнал «Медицина неотложных состояний» Том 21, №6, 2025
Вернуться к номеру
Три контури захисту мітохондрій при гострому панкреатиті: біоенергетика, редокс-баланс і блокада пори перехідної проникності
Авторы: Чуклін С.М., Чуклін С.С.
Медичний центр Святої Параскеви, м. Львів, Україна
Рубрики: Медицина неотложных состояний
Разделы: Справочник специалиста
Версия для печати
Мітохондріальна дисфункція є ключовою патогенетичною ланкою гострого панкреатиту (ГП), спричиняючи порушення вироблення АТФ, втрату мембранного потенціалу (m), утворення активних форм кисню (АФК) та відкриття пори перехідної проникності (mPTP), що призводить до некрозу ацинарних клітин і системної запальної відповіді. Це обґрунтовує доцільність терапевтичного втручання, спрямованого на стабілізацію мітохондріального гомеостазу. Мета: систематизувати сучасні експериментальні підходи до мітохондріально-спрямованої терапії при ГП, зосереджені на трьох напрямках: підтримці мітохондріального біоенергетичного гомеостазу, антиоксидантній дії та інгібуванні відкриття mPTP. Проведено аналіз оригінальних досліджень із баз даних PubMed, Scopus і Google Scholar, присвячених впливу фармакологічних агентів на функцію мітохондрій в експериментальних моделях ГП. Оцінювалися біоенергетичні параметри (синтез АТФ, m), рівні окисного стресу, активація сигнальних шляхів (SIRT1, Nrf2, HtrA2, PGC-1), ступінь некрозу, інтенсивність запальної відповіді й активність mPTP. Засоби, що підтримують енергетичну функцію мітохондрій (карнітин, NMN, еламіпретид, коензим Q10, мелатонін), сприяють збереженню m, стимуляції ЦТК та поліпшенню виживання клітин. Антиоксиданти (куркумін, гідрокситирозол, кверцетин, астрагалозид IV, тирон) демонструють здатність зменшувати АФК-залежне ушкодження, активуючи внутрішньоклітинні шляхи Nrf2/ARE. Інгібітори mPTP (циклоспорин, SS-31) запобігають відкриттю пори, зменшуючи втрату m і некроз. MitoTEMPO і HRS діють насамперед як таргетні антиоксиданти, знижуючи рівень мтАФК, що, як наслідок, супроводжується пригніченням інфламасомної активації. Водночас деякі агенти (MitoQ, SkQ1) давали несприятливі ефекти або погіршення перебігу. Мітохондріальна таргетна терапія є перспективним напрямом експериментального лікування ГП. Засоби, які відновлюють біоенергетичну функцію, зменшують оксидативний стрес і блокують відкриття mPTP, забезпечують морфофункціональний захист ацинарних клітин. Подальші дослідження мають бути спрямовані на клінічну апробацію найефективніших агентів та оптимізацію комбінованих підходів.
Mitochondrial dysfunction plays a central role in the pathogenesis of acute pancreatitis (AP), leading to impaired adenosine triphosphate (ATP) production, loss of mitochondrial membrane potential (m), excessive reactive oxygen species (ROS) formation, and opening of the mitochondrial permeability transition pore (mPTP), which drives acinar cell necrosis and systemic inflammation. These mechanisms highlight mitochondria as a promising therapeutic target. The purpose was to summarize experimental strategies for mitochondria-targeted therapy in AP, focusing on three major aspects: support of bioenergetic homeostasis, antioxidant action, and inhibition of mPTP opening. A structured analysis of original experimental studies from PubMed, Scopus, and Google Scholar was conducted. Studies examined the effects of mitochondria-targeted agents on ATP synthesis, m stabilization, oxidative stress, regulatory pathways (SIRT1, Nrf2, HtrA2, PGC-1), necrosis, inflammatory response, and mPTP regulation in AP models. Energy-supporting agents (e.g., carnitine, NMN, elamipretide, coenzyme Q10, melatonin) enhanced mitochondrial function and acinar cell viability. Antioxidants (e.g., curcumin, hydroxytyrosol, quercetin, astragaloside IV, tiron) reduced ROS-mediated injury via Nrf2/ARE pathway activation. mPTP inhibitors (cyclosporine, SS-31) preserved m and reduced necrosis. MitoTEMPO and hydrogen-rich saline primarily acted as mitochondria-targeted antioxidants, reducing ROS levels, which secondarily resulted in suppression of inflammasome activation. In contrast, MitoQ and SkQ1 showed unfavorable or inconsistent results. Mitochondria-targeted therapy is a promising approach to experimental AP treatment. Agents that support mitochondrial energetics, reduce oxidative stress, and inhibit mPTP opening offer cytoprotective benefits and may improve disease outcomes. Future research should focus on clinical validation and combined therapeutic strategies.
гострий панкреатит; мітохондріальна дисфункція; мітохондріальна таргетна терапія; огляд
acute pancreatitis; mitochondrial dysfunction; mitochondrial targeted therapy; review
Для ознакомления с полным содержанием статьи необходимо оформить подписку на журнал.
- Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA. 2021;325(4):382-390. doi: 10.1001/jama.2020.20317. PMID: 33496779.
- Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396(10252):726-734. doi: 10.1016/S0140-6736(20)31310-6. PMID: 32891214.
- Shah J, Fernandez Y Viesca M, Jagodzinski R, Arvanitakis M. Infected pancreatic necrosis-Current trends in management. Indian J Gastroenterol. 2024;43(3):578-591. doi: 10.1007/s12664-023-01506-w. Epub 2024 Apr 16. PMID: 38625518.
- Li M, Wu L, Si H, Wu Y, Liu Y, Zeng Y, Shen B. Engineered mitochondria in diseases: mechanisms, strategies, and applications. Signal Transduct Target Ther. 2025;10(1):71. doi: 10.1038/s41392-024-02081-y. PMID: 40025039.
- Moura JP, Oliveira PJ, Urbano AM. Mitochondria: An overview of their origin, genome, architecture, and dynamics. Biochim Biophys Acta Mol Basis Dis. 2025;1871(5):167803. doi: 10.1016/j.bbadis.2025.167803. Epub 2025 Mar 19. PMID: 40118291.
- Li Y, Zhang H, Yu C, Dong X, Yang F, Wang M, et al. New Insights into Mitochondria in Health and Diseases. Int J Mol Sci. 2024;25(18):9975. doi: 10.3390/ijms25189975. PMID: 39337461.
- Xu M, Feng Y, Xiang X, Liu L, Tang G. MZB1 regulates cellular proliferation, mitochondrial dysfunction, and inflammation and targets the PI3K-Akt signaling pathway in acute pancreatitis. Cell Signal. 2024;118:111143. doi: 10.1016/j.cellsig.2024.111143. Epub 2024 Mar 18. PMID: 38508349.
- Liu W, Ren Y, Wang T, Wang M, Xu Y, Zhang J, et al. Bloc–king CIRP protects against acute pancreatitis by improving mitochondrial function and suppressing pyroptosis in acinar cells. Cell Death Discov. 2024;10(1):156. doi: 10.1038/s41420-024-01923-6. PMID: 38538578.
- Zhang D, Li J, Zhao L, Yang Z, Wu C, Liu Y, et al. Mitochondrial DNA Leakage Promotes Persistent Pancreatic Acinar Cell Injury in Acute Pancreatitis via the cGAS-STING-NF-B Pathway. Inflammation. 2024 Aug 24. doi: 10.1007/s10753-024-02132-0. Online ahead of print. PMID: 39180578.
- Pandol SJ, Gottlieb RA. Calcium, mitochondria and the initiation of acute pancreatitis. Pancreatology. 2022;22(7):838-845. doi: 10.1016/j.pan.2022.07.011. Epub 2022 Aug 3. PMID: 35941013.
- Chen F, Xu K, Han Y, Ding J, Ren J, Wang Y, et al. Mitochondrial dysfunction in pancreatic acinar cells: mechanisms and therapeutic strategies in acute pancreatitis. Front Immunol. 2024;15:1503087. doi: 10.3389/fimmu.2024.1503087. eCollection 2024. PMID: 39776917.
- Chen X, Zhong R, Hu B. Mitochondrial dysfunction in the pathogenesis of acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2025;24(1):76-83. doi: 10.1016/j.hbpd.2023.12.008. Epub 2023 Dec 30. PMID: 38212158.
- Zhou Y, Huang X, Jin Y, Qiu M, Ambe PC, Basharat Z, Hong W. The role of mitochondrial damage-associated molecular patterns in acute pancreatitis. Biomed Pharmacother. 2024;175:116690. doi: 10.1016/j.biopha.2024.116690. Epub 2024 May 7. PMID: 38718519.
- Hong WL, Huang H, Zeng X, Duan CY. Targeting mitochondrial quality control: new therapeutic strategies for major diseases. Mil Med Res. 2024;11(1):59. doi: 10.1186/s40779-024-00556-1. PMID: 39164792.
- Wang L, Zhou X, Lu T. Role of mitochondria in physiological activities, diseases, and therapy. Mol Biomed. 2025;6(1):42. doi: 10.1186/s43556-025-00284-5. PMID: 40536597.
- Soliman GF, Ibrahim W, Abdallah H. Therapeutic Effect of L-Carnitine on Acute Pancreatitis Induced by L-Arginine in Rats: Possible Role of Beclin Gene and Inducible Nitric Oxide Synthase. Med J Cairo Univ. 2019;87(3):1793-1803.
- Karakahya M, Gl M, Ik S, Aydn C, Yiitcan B, Otan E, Orug T. The histopathologic effects of L-carnitine in Sodium Taurocholate Induced Severe Pancreatitis Model. Int Surg. 2016;101(5-6):241-248. doi: 10.9738/INTSURG-D-16-00058.1. PMID: 27119771.
- Hasan MIA, Bakr AG, Shalkami AS. Modulation of L-arginine-induced acute pancreatitis by meloxicam and/or L-carnitine in rats. Int J Basic Clin Pharmacol. 2015;4:1247-1253. doi: 10.18203/2319-2003.ijbcp20151367.
- McIlwrath SL, Starr ME, High AE, Saito H, Westlund KN. Effect of acetyl-L-carnitine on hypersensitivity in acute recurrent caerulein-induced pancreatitis and microglial activation along the brain’s pain circuitry. World J Gastroenterol. 2021;27(9):794-814. doi: 10.3748/wjg.v27.i9.794. PMID: 33727771.
- Duan H, Zhang R, Asikaer A, Pan L, Wang S, Huang K, et al. Nicotinamide mononucleotide ameliorates hypertriglyceridemia pancreatitis via NAD+/SIRT1-mediated TXNIP suppression and NOTCH pathway for accelerated repair-associated processes. Int Immunopharmacol. 2025 May 16;155:114620. doi: 10.1016/j.intimp.2025.114620. Epub 2025 Apr 10. PMID: 40215777.
- Shen A, Kim HJ, Oh GS, Lee SB, Lee SH, Pandit A, et al. NAD+ augmentation ameliorates acute pancreatitis through regulation of inflammasome signalling. Sci Rep. 2017;7(1):3006. doi: 10.1038/s41598-017-03418-0. PMID: 28592850.
- Liao WC, Chen YH, Li HY, Wang TT, Lan P, Pan KH, et al. Diaphragmatic dysfunction in sepsis due to severe acute pancreatitis complicated by intra-abdominal hypertension. J Int Med Res. 2018;46(4):1349-1357. doi: 10.1177/0300060517747163. Epub 2018 Jan 29.PMID: 29376467.
- Mirmalek SA, Gholamrezaei Boushehrinejad A, Yavari H, Kardeh B, Parsa Y, Salimi-Tabatabaee SA, et al. Antioxidant and Anti-Inflammatory Effects of Coenzyme Q10 on L-Arginine-Induced Acute Pancreatitis in Rat. Oxid Med Cell Longev. 2016;2016:5818479. doi: 10.1155/2016/5818479. Epub 2016 Apr 12. PMID: 27190575.
- Elsyade RH, Abdel Fattah MM, Khaled DM, Eldosoki DE. The Beneficial Effects of Lipoic Acid Versus Coenzyme Q10 on Arginine-Induced Acute Pancreatitis in Albino Rats: Histological and Immunohistochemical study. J Med Histology. 2021;5(2):154-170. doi: 10.21608/jmh.2022.161806.1103.
- Shin JY, Choi JW, Kim DG, Zhou ZQ, Shin YK, Seo JH, et al. Protective effects of Coenzyme Q10 against acute pancreatitis. Int Immunopharmacol. 2020;88:106900. doi: 10.1016/j.intimp.2020.106900. Epub 2020 Aug 20. PMID: 32829089.
- Li Y, Zhu Y, Li S, Dong Y, Wan C, Yu X, et al. Deoxyarbutin attenuates severe acute pancreatitis via the HtrA2/PGC-1 pathway. Free Radic Res. 2022;56(9-10):651-665. doi: 10.1080/10715762.2022.2163244. Epub 2023 Jan 2. PMID: 36592372.
- Zhao T, Fang R, Ding J, Liu Y, Cheng M, Zhou F, et al. Melatonin ameliorates multiorgan injuries induced by severe acute pancreatitis in mice by regulating the Nrf2 signaling pathway. Eur J Pharmacol. 202415;975:176646. doi: 10.1016/j.ejphar.2024.176646. Epub 2024 May 17. PMID: 38762157.
- Alruhaimi RS, Hassanein EHM, Abd El-Aziz MK, Siddiq Abduh M, Bin-Ammar A, Kamel EM, Mahmoud AM. The melatonin receptor agonist agomelatine protects against acute pancreatitis induced by cadmium by attenuating inflammation and oxidative stress and modulating Nrf2/HO-1 pathway. Int Immunopharmacol. 2023 Nov;124(Pt A):110833. doi: 10.1016/j.intimp.2023.110833. Epub 2023 Aug 25. PMID: 37634447.
- Uluk D, Keskin E, zaydn T, znurlu Y. Ameliorative effects of the melatonin on some cytokine levels, NF-B immunoreactivity, and apoptosis in rats with cerulein-induced acute pancreatitis. Iran J Basic Med Sci. 2024;27(3):279-285. doi: 10.22038/IJBMS.2023.69019.15045. PMID: 38333760.
- Ren Y, Qiu M, Zhang J, Bi J, Wang M, Hu L, Du Z, Li T, et al. Low Serum Irisin Concentration Is Associated with Poor Outcomes in Patients with Acute Pancreatitis, and Irisin Administration Protects Against Experimental Acute Pancreatitis. Antioxid Redox Signal. 2019;31(11):771-785. doi: 10.1089/ars.2019.7731. Epub 2019 Jul 29. PMID: 31250660.
- Tth E, Malth J, Zvogyn N, Fanczal J, Grassalkovich A, Erds R, et al. Novel mitochondrial transition pore inhibitor N-methyl-4-isoleucine cyclosporin is a new therapeutic option in acute pancreatitis. J Physiol. 2019;597(24):5879-5898. doi: 10.1113/JP278517. Epub 2019 Dec 1. PMID: 31631343.
- Bulut NE, zkan E, Ekinci O, Dulundu E, Topalolu , ehirli A, et al. Beneficial effects of alpha lipoic acid on cerulein-induced experimental acute pancreatitis in rats. Ulus Travma Acil Cerrahi Derg. 2011;17(5):383-9. PMID: 22090321.
- Park SJ, Seo SW, Choi OS, Park CS. Alpha-lipoic acid protects against cholecystokinin-induced acute pancreatitis in rats. World J Gastroenterol. 2005;11(31):4883-5. doi: 10.3748/wjg.v11.i31.4883. PMID: 16097064.
- Abdin AA, El-Hamid MA, El-Seoud SH, Balaha MF. Effect of pentoxifylline and/or alpha lipoic acid on experimentally induced acute pancreatitis. Eur J Pharmacol. 2010;643(2-3):289-96. doi: 10.1016/j.ejphar.2010.06.020. Epub 2010 Jun 21. PMID: 20599924.
- Lee Y, Lim JW, Kim H. lipoic acid inhibits cerulein/resistin induced expression of interleukin 6 by activating peroxisome proliferator activated receptor in pancreatic acinar cells. Mol Med Rep. 2022;26(2):264. doi: 10.3892/mmr.2022.12780. Epub 2022 Jun 22.PMID: 35730599.
- Fawzy H, Fikry E, Fawzy H, Mohammed A. Mito-TEMPO improved L-Arginine-induced acute pancreatitis in rats via TLR-4/NF-кB/NLRP3 inflammasome downregulation and antioxidant properties. Azhar Int J Pharm Med Sci. 2021;1(1):54-65. doi: 10.21608/aijpms.2021.54059.1026.
- Wang X, Guo Y, Cui T, Zhang T, Hu W, Liu R, Yin C. Telo–merase reverse transcriptase restores pancreatic microcirculation profiles and attenuates endothelial dysfunction by inhibiting mitochondrial superoxide production: A potential target for acute pancreatitis therapy. Biomed Pharmacother. 2023;167:115576. doi: 10.1016/j.biopha.2023.115576. Epub 2023 Sep 28. PMID: 37776643.
- Luo ZL, Sun HY, Wu XB, Cheng L, Ren JD. Epigalloca–techin-3-gallate attenuates acute pancreatitis induced lung injury by targeting mitochondrial reactive oxygen species triggered NLRP3 inflammasome activation. Food Funct. 2021;12(12):5658-5667. doi: 10.1039/d1fo01154e. PMID: 34018522.
- Rius-Prez S, Prez S, Toledano MB, Sastre J. p53 drives necroptosis via downregulation of sulfiredoxin and peroxiredoxin 3. Redox Biol. 2022;56:102423. doi: 10.1016/j.redox.2022.102423. Epub 2022 Aug 20. PMID: 36029648.
- Huang W, Cash N, Wen L, Szatmary P, Mukherjee R, Armstrong J, et al. Effects of the mitochondria-targeted antioxidant mitoquinone in murine acute pancreatitis. Mediators Inflamm. 2015;2015:901780. doi: 10.1155/2015/901780. Epub 2015 Mar 23. PMID: 258784.
- Armstrong JA, Cash NJ, Morton JC, Tepikin AV, Sutton R, Criddle DN. Mitochondrial Targeting of Antioxidants Alters Pancreatic Acinar Cell Bioenergetics and Determines Cell Fate. Int J Mol Sci. 2019;20(7):1700. doi: 10.3390/ijms20071700.PMID: 30959771.
- Weniger M, Reinelt L, Neumann J, Holdt L, Ilmer M, Renz B, et al. The Analgesic Effect of the Mitochondria-Targeted Antioxidant SkQ1 in Pancreatic Inflammation. Oxid Med Cell Longev. 2016;2016:4650489. doi: 10.1155/2016/4650489. Epub 2016 May 4. PMID: 27274778.
- Lee J, Lim JW, Kim H. Lycopene Inhibits IL-6 Expression by Upregulating NQO1 and HO-1 via Activation of Nrf2 in Ethanol/Lipopolysaccharide-Stimulated Pancreatic Acinar Cells. Antioxidants (Basel). 2022;11(3):519. doi: 10.3390/antiox11030519. PMID: 35326169.
- Lee J, Lim JW, Kim H. Lycopene Inhibits Oxidative Stress-Mediated Inflammatory Responses in Ethanol/Palmito–leic Acid-Sti–mulated Pancreatic Acinar AR42J Cells. Int J Mol Sci. 2021;22(4):2101. doi: 10.3390/ijms22042101. PMID: 33672594.
- Ateyya H, Wagih HM, El-Sherbeeny NA. Effect of tiron on remote organ injury in rats with severe acute pancreatitis induced by L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 2016 Aug;389(8):873-85. doi: 10.1007/s00210-016-1250-6. Epub 2016 Apr 27.PMID: 27118662.
- Chen H, Sun YP, Li Y, Liu WW, Xiang HG, Fan LY, Sun Q, et al. Hydrogen-rich saline ameliorates the severity of l-arginine-induced acute pancreatitis in rats. Biochem Biophys Res Commun. 2010;393(2):308-13. doi: 10.1016/j.bbrc.2010.02.005. Epub 2010 Feb 6. PMID: 20138831.
- Zhang DQ, Feng H, Chen WC. Effects of hydrogen-rich saline on taurocholate-induced acute pancreatitis in rat. Evid Based Complement Alternat Med. 2013;2013:731932. doi: 10.1155/2013/731932. Epub 2013 Jul 28. PMID: 23983797.
- Ren JD, Ma J, Hou J, Xiao WJ, Jin WH, Wu J, Fan KH. Hydrogen-rich saline inhibits NLRP3 inflammasome activation and attenuates experimental acute pancreatitis in mice. Mediators Inflamm. 2014;2014:930894. doi: 10.1155/2014/930894. Epub 2014 Aug 20. PMID: 25214720.
- Shi Q, Liao KS, Zhao KL, Wang WX, Zuo T, Deng WH, et al. Hydrogen-rich saline attenuates acute renal injury in sodium taurocholate-induced severe acute pancreatitis by inhibiting ROS and NF-B pathway. Mediators Inflamm. 2015;2015:685043. doi: 10.1155/2015/685043. Epub 2015 Mar 23.PMID: 25878401.
- Shi Q, Chen C, Deng WH, Wang P, Zuo T, Zhao L, et al. Hydrogen-Rich Saline Attenuates Acute Hepatic Injury in Acute Necrotizing Pancreatitis by Inhibiting Inflammation and Apoptosis, Involving JNK and p38 Mitogen-Activated Protein Kinase-dependent Reactive Oxygen Species. Pancreas. 2016;45(10):1424-1431. doi: 10.1097/MPA.0000000000000678. PMID: 27518466.
- Ren J, Luo Z, Tian F, Wang Q, Li K, Wang C. Hydrogen-rich saline reduces the oxidative stress and relieves the severity of trauma-induced acute pancreatitis in rats. J Trauma Acute Care Surg. 2012;72(6):1555-61. doi: 10.1097/TA.0b013e31824a7913. PMID: 22695421.
- Li Y, Li G, Suo L, Zhang J. Recent advances in stu–dies of molecular hydrogen in the treatment of pancreatitis. Life Sci. 2021;264:118641. doi: 10.1016/j.lfs.2020.118641. Epub 2020 Oct 24. PMID: 33148420.
- Dong Z, Shang H, Chen YQ, Pan LL, Bhatia M, Sun J. Sulforaphane Protects Pancreatic Acinar Cell Injury by Modulating Nrf2-Mediated Oxidative Stress and NLRP3 Inflammatory Pathway. Oxid Med Cell Longev. 2016;2016:7864150. doi: 10.1155/2016/7864150. Epub 2016 Oct 26. PMID: 27847555.
- Zhang P, Yin X, Wang X, Wang J, Na G, Короткова И. Paeonol protects against acute pancreatitis by Nrf2 and NF-B pathways in mice. J Pharm Pharmacol. 2022;74(11):1618-1628. doi: 10.1093/jpp/rgac065. PMID: 36170125.
- Cui Q, Wang W, Shi J, Lai F, Luo S, Du Y, Wang X, Xiang Y. Glycyrrhizin Ameliorates Cardiac Injury in Rats with Severe Acute Pancreatitis by Inhibiting Ferroptosis via the Keap1/Nrf2/HO-1 Pathway. Dig Dis Sci. 2024;69(7):2477-2487. doi: 10.1007/s10620-024-08398-6. Epub 2024 May 16. PMID: 38753240.
- Shen G, Wen H, Li H, Zhang X, Lan B, Dong X, et al. Emodin protects against severe acute pancreatitis-associated acute lung injury by activating Nrf2/HO-1/GPX4 signal and inhibiting ferroptosis in vivo and in vitro. BMC Gastroenterol. 2025;25(1):57. doi: 10.1186/s12876-025-03660-1. PMID: 39910464.
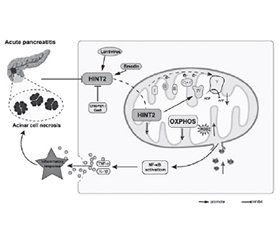
- Yao J, Jiang Y, Zhang P, Miao Y, Wu X, Lei H, et al. Genetic and pharmacological targeting of HINT2 promotes OXPHOS to alleviate inflammatory responses and cell necrosis in acute pancreatitis. Pharmacol Res. 2025;212:107620. doi: 10.1016/j.phrs.2025.107620. Epub 2025 Jan 21. PMID: 3984835.
- Sheng B, Zhao L, Zang X, Zhen J, Liu Y, Bian W, Chen W. Quercetin inhibits caerulein-induced acute pancreatitis through regulating miR-216b by targeting MAP2K6 and NEAT1. Inflammopharmacology. 2021 Apr;29(2):549-559. doi: 10.1007/s10787-020-00767-7. Epub 2020 Oct 13. PMID: 33051781.
- Junyuan Z, Hui X, Chunlan H, Junjie F, Qixiang M, Yingying L, et al. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38 MAPK and ERS inhibition. Pancreatology. 2018 Oct;18(7):742-752. doi: 10.1016/j.pan.2018.08.001. Epub 2018 Aug 6. PMID: 30115563.
- Li X, Wang T, Zhou Q, Li F, Liu T, Zhang K, Wen A, et al. Isorhamnetin Alleviates Mitochondrial Injury in Severe Acute Pancreatitis via Modulation of KDM5B/HtrA2 Signaling Pathway. Int J Mol Sci. 2024;25(7):3784. doi: 10.3390/ijms25073784. PMID: 38612598.
- Fusco R, Cordaro M, Siracusa R, D’Amico R, Genovese T, Gugliandolo E, et al. Biochemical Evaluation of the Antioxidant Effects of Hydroxytyrosol on Pancreatitis-Associated Gut Injury. Antioxidants (Basel). 2020;9(9):781. doi: 10.3390/antiox9090781. PMID: 32842687.
- Siriviriyakul P, Chingchit T, Klaikeaw N, Chayanupatkul M, Werawatganon D. Effects of curcumin on oxidative stress, inflammation and apoptosis in L-arginine induced acute pancreatitis in mice. Heliyon. 2019;5(8):e02222. doi: 10.1016/j.heliyon.2019.e02222. eCollection 2019 Aug. PMID: 31485503.
- Wang Y, Bu C, Wu K, Wang R, Wang J. Curcumin protects the pancreas from acute pancreatitis via the mitogen activated protein kinase signaling pathway. Mol Med Rep. 2019;20(4):3027-3034. doi: 10.3892/mmr.2019.10547. Epub 2019 Aug 1. PMID: 31432122.
- Anchi P, Khurana A, Swain D, Samanthula G, Godugu C. Sustained-Release Curcumin Microparticles for Effective Prophylactic Treatment of Exocrine Dysfunction of Pancreas: A Preclinical Study on Cerulein-Induced Acute Pancreatitis. J Pharm Sci. 2018;107(11):2869-2882. doi: 10.1016/j.xphs.2018.07.009. Epub 2018 Jul 19. PMID: 30031026.
- Zhu S, Zhang C, Weng Q, Ye B. Curcumin protects against acute renal injury by suppressing JAK2/STAT3 pathway in severe acute pancreatitis in rats. Exp Ther Med. 2017;14(2):1669-1674. doi: 10.3892/etm.2017.4647. Epub 2017 Jun 22. PMID: 28810635.
- Yu S, Wang M, Guo X, Qin R. Curcumin Attenuates Inflammation in a Severe Acute Pancreatitis Animal Model by Regulating TRAF1/ASK1 Signaling. Med Sci Monit. 2018;24:2280-2286. doi: 10.12659/msm.909557. PMID: 29657313.
- Chegini M, Sadeghi A, Zaeri F, Zamani M, Hekmatdoost A. Nano-curcumin supplementation in patients with mild and moderate acute pancreatitis: A randomized, placebo-controlled trial. Phytother Res. 2023;37(11):5279-5288. doi: 10.1002/ptr.7958. Epub 2023 Jul 25. PMID: 37490939.
- Ji L, Li L, Qu F, Zhang G, Wang Y, Bai X, Pan S, Xue D, Wang G, Sun B. Hydrogen sulphide exacerbates acute pancreatitis by over-activating autophagy via AMPK/mTOR pathway. J Cell Mol Med. 2016;20(12):2349-2361. doi: 10.1111/jcmm.12928. Epub 2016 Jul 15. PMID: 27419805.
- Salam K, Alhan E, Trkylmaz S, Vanizor BK, Erin C. The anti-inflammatory effect of hydrogen sulphide on acute necrotizing pancreatitis in rats. Turk J Surg. 2017;33(3):158-163. doi: 10.5152/UCD.2017.3653. eCollection 2017. PMID: 28944326.
- Rao CY, Fu LY, Hu CL, Chen DX, Gan T, Wang YC, Zhao XY. H2S mitigates severe acute pancreatitis through the PI3K/AKT-NF-B pathway in vivo. World J Gastroenterol. 2015;21(15):4555-63. doi: 10.3748/wjg.v21.i15.4555. PMID: 25914464.
- Qiu L, Yin G, Cheng L, Fan Y, Xiao W, Yu G, et al. Astragaloside IV ameliorates acute pancreatitis in rats by inhibiting the activation of nuclear factor-B. Int J Mol Med. 2015 Mar;35(3):625-36. doi: 10.3892/ijmm.2015.2070. Epub 2015 Jan 16. PMID: 25604657.
- Yuan J, Wei Z, Xin G, Liu X, Zhou Z, Zhang Y, et al. Vitamin B12 Attenuates Acute Pancreatitis by Suppressing Oxidative Stress and Improving Mitochondria Dysfunction via CBS/SIRT1 Pathway. Oxid Med Cell Longev. 2021;2021:7936316. doi: 10.1155/2021/7936316. eCollection 2021. PMID: 34925701.
- Liang QQ, Shi ZJ, Yuan T, Chen SY, Li YP, Zhang HR, et al. Celastrol inhibits necroptosis by attenuating the RIPK1/RIPK3/MLKL pathway and confers protection against acute pancreatitis in mice. Int Immunopharmacol. 2023;117:109974. doi: 10.1016/j.intimp.2023.109974. Epub 2023 Mar 8. PMID: 37012867.
- Luo S, Li P, Li S, Du Z, Hu X, Fu Y, Zhang Z. N,N-Dimethyl Tertiary Amino Group Mediated Dual Pancreas- and Lung-Targeting Therapy against Acute Pancreatitis. Mol Pharm. 2017;14(5):1771-1781. doi: 10.1021/acs.molpharmaceut.7b00028. Epub 2017 Mar 27. PMID: 28247763.
- Armstrong JA, Cash NJ, Ouyang Y, Morton JC, Chvanov M, Latawiec D, et al. Oxidative stress alters mitochondrial bioenergetics and modifies pancreatic cell death independently of cyclophilin D, resulting in an apoptosis-to-necrosis shift. J Biol Chem. 2018;293(21):8032-8047. doi: 10.1074/jbc.RA118.003200. Epub 2018 Apr 6. PMID: 29626097.
- Shen Y, Wen L, Zhang R, Wei Z, Shi N, Xiong Q, et al. Dihydrodiosgenin protects against experimental acute pancreatitis and associated lung injury through mitochondrial protection and PI3K/Akt inhibition. Br J Pharmacol. 2018;175(10):1621-1636. doi: 10.1111/bph.14169. Epub 2018 Apr 2. PMID: 29457828.
- Zhang C, Niu H, Wan C, Yu X, Xin G, Zhu Y, et al. Drug D, a Diosgenin Derive, Inhibits L-Arginine-Induced Acute Pancreatitis through Meditating GSDMD in the Endoplasmic Reticulum via the TXNIP/HIF-1 Pathway. Nutrients. 2022;14(13):2591. doi: 10.3390/nu14132591. PMID: 35807771.
- Javed MA, Wen L, Awais M, Latawiec D, Huang W, Chvanov M, et al. TRO40303 Ameliorates Alcohol-Induced Pancreatitis Through Reduction of Fatty Acid Ethyl Ester-Induced Mitochondrial Injury and Necrotic Cell Death. Pancreas. 2018;47(1):18-24. doi: 10.1097/MPA.0000000000000953. PMID: 29200128.
- Yu X, Dai C, Zhao X, Huang Q, He X, Zhang R, et al. Ruthenium red attenuates acute pancreatitis by inhibiting MCU and improving mitochondrial function. Biochem Biophys Res Commun. 2022;635:236-243. doi: 10.1016/j.bbrc.2022.10.044. Epub 2022 Oct 14. PMID: 3628333.
- Lei Y, Yang HY, Meng N, Qin YY, Xu MT, Xiang XL, et al. Mitochondrial calcium uniporter promotes mitophagy by regulating the PINK1/Parkin pathway in caerulein treated pancreatic ductal epithelial cells in vitro. Exp Ther Med. 2024;27(4):147. doi: 10.3892/etm.2024.12435. eCollection 2024 Apr. PMID: 38476889.